SARS-CoV-2 S Protein Reduces Cytoprotective Defenses and Promotes Human Endothelial Cell Senescence
- PMID: 39012668
- PMCID: PMC12096926
- DOI: 10.14336/AD.2024.0405
SARS-CoV-2 S Protein Reduces Cytoprotective Defenses and Promotes Human Endothelial Cell Senescence
Abstract
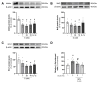
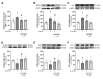
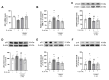
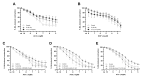
Premature vascular aging and endothelial cell senescence are major risk factors for cardiovascular diseases and atherothrombotic disturbances, which are main complications of both acute and long COVID-19. The S protein of SARS-CoV2, which acts as the receptor binding protein for the viral infection, is able to induce endothelial cells inflammation and it has been found as an isolated element in the circulation and in human tissues reservoirs months after infection. Here, we investigated whether the S protein is able to directly induce endothelial cell senescence and deciphered some of the mechanisms involved. In primary cultures of human umbilical vein endothelial cells (HUVEC), SARS-CoV-2 S protein enhanced in a concentration-dependent manner the cellular content of senescence and DNA damage response markers (senescence-associated-β galactosidase, γH2AX), as well as growth-arrest effectors (p53, p21, p16). In parallel, the S protein reduced the availability of cytoprotective proteins, such as the anti-aging protein klotho, Nrf2 or heme oxygenase-1, and caused functional harm by impairing ex vivo endothelial-dependent vasorelaxation in murine microvessels. These effects were prevented by the pharmacological inhibition of the NLRP3 inflammasome with MCC950. Furthermore, the supplementation with either recombinant klotho or angiotensin-(1-7), equally protected against the pro-senescence, pro-inflammatory and pro-oxidant action of the S protein. Globally, this study proposes novel mechanisms of disease in the context of COVID-19 and its vascular sequelae and provides pharmacological clues in order to prevent such complications.
Figures


References
-
- Saz-Lara A, Cavero-Redondo I, Pascual-Morena C, Martínez-García I, Rodríguez-Gutiérrez E, Lucerón-Lucas-Torres M, et al.. (2023). Early vascular aging as an index of cardiovascular risk in healthy adults: confirmatory factor analysis from the EVasCu study. Cardiovasc Diabetol, 22:1-8. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
