DRD2 activation inhibits choroidal neovascularization in patients with Parkinson's disease and age-related macular degeneration
- PMID: 39012703
- PMCID: PMC11364393
- DOI: 10.1172/JCI174199
DRD2 activation inhibits choroidal neovascularization in patients with Parkinson's disease and age-related macular degeneration
Abstract
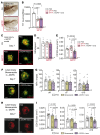
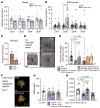
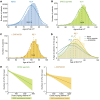
Neovascular age-related macular degeneration (nAMD) remains a major cause of visual impairment and puts considerable burden on patients and health care systems. l-DOPA-treated Parkinson's disease (PD) patients have been shown to be partially protected from nAMD, but the mechanism remains unknown. Using murine models that combine 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced (MPTP-induced) PD and laser-induced nAMD with standard PD treatment of l-DOPA/DOPA-decarboxylase inhibitor or specific dopamine receptor inhibitors, we here demonstrate that l-DOPA treatment-induced increase of dopamine-mediated dopamine receptor D2 (DRD2) signaling inhibits choroidal neovascularization independently of MPTP-associated nigrostriatal pathway lesion. Analyzing a retrospective cohort of more than 200,000 patients with nAMD receiving anti-VEGF treatment from the French nationwide insurance database, we show that DRD2 agonist-treated PD patients have a significantly delayed age of onset of nAMD and reduced need for anti-VEGF therapies, similar to the effects of the l-DOPA treatment. While providing a mechanistic explanation for an intriguing epidemiological observation, our findings suggest that systemic DRD2 agonists might constitute an adjuvant therapy to delay and reduce the need for anti-VEGF therapy in patients with nAMD.
Keywords: Neurodegeneration; Neuroscience; Ophthalmology; Parkinson disease; Retinopathy.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

