Setrusumab for the treatment of osteogenesis imperfecta: 12-month results from the phase 2b asteroid study
- PMID: 39012717
- PMCID: PMC11371902
- DOI: 10.1093/jbmr/zjae112
Setrusumab for the treatment of osteogenesis imperfecta: 12-month results from the phase 2b asteroid study
Erratum in
-
Correction to: Setrusumab for the treatment of osteogenesis imperfecta: 12-month results from the phase 2b asteroid study.J Bone Miner Res. 2025 Apr 21;40(4):561. doi: 10.1093/jbmr/zjaf031. J Bone Miner Res. 2025. PMID: 40037367 Free PMC article. No abstract available.
Abstract
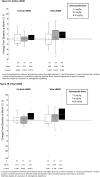
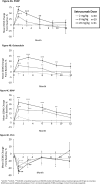
Osteogenesis imperfecta (OI) is a rare genetic disorder commonly caused by variants of the type I collagen genes COL1A1 and COL1A2. OI is associated with increased bone fragility, bone deformities, bone pain, and reduced growth. Setrusumab, a neutralizing antibody to sclerostin, increased areal bone mineral density (aBMD) in a 21-week phase 2a dose escalation study. The phase 2b Asteroid (NCT03118570) study evaluated the efficacy and safety of setrusumab in adults. Adults with a clinical diagnosis of OI type I, III, or IV, a pathogenic variant in COL1A1/A2, and a recent fragility fracture were randomized 1:1:1:1 to receive 2, 8, or 20 mg/kg setrusumab doses or placebo by monthly intravenous infusion during a 12-mo treatment period. Participants initially randomized to the placebo group were subsequently reassigned to receive setrusumab 20 mg/kg open label. Therefore, only results from the 2, 8, and 20 mg/kg double-blind groups are presented herein. The primary endpoint of Asteroid was change in distal radial trabecular volumetric bone mineral density (vBMD) from baseline at month 12, supported by changes in high-resolution peripheral quantitative computed tomography micro-finite element (microFE)-derived bone strength. A total of 110 adults were enrolled with similar baseline characteristics across treatment groups. At 12 mo, there was a significant increase in mean (SE) failure load in the 20 mg/kg group (3.17% [1.26%]) and stiffness in the 8 (3.06% [1.70%]) and 20 mg/kg (3.19% [1.29%]) groups from baseline. There were no changes in radial trabecula vBMD (p>05). Gains in failure load and stiffness were similar across OI types. There were no significant differences in annualized fracture rates between doses. Two adults in the 20 mg/kg group experienced related serious adverse reactions. Asteroid demonstrated a beneficial effect of setrusumab on estimates of bone strength across the different types of OI and provides the basis for additional phase 3 evaluation.
Keywords: bone mineral density; osteogenesis imperfecta; rare disease; sclerostin; setrusumab.
Plain language summary
Osteogenesis imperfecta (OI), is a rare disorder affecting patients’ bones causing pain and an increased chance of the bone breaking. Setrusumab is a possible treatment for OI being studied in a clinical trial called Asteroid. The goal of Asteroid was to determine which dose of setrusumab helped adults with OI the most: 2, 8, or 20 mg/kg. Researchers looked at the density of patients’ bones and estimated how strong their bones were before setrusumab and again after 12 mo of treatment to see how they improved with treatment. Researchers could compare these improvements to see which dose of setrusumab helped patients the most. Patients on the highest dose of setrusumab (20 mg/kg) experienced improvements in the density of their arm bones (radius) and leg bones (tibia) after 12 mo. The strength of these bones also improved. The density of other bones including the spine, hip, and the overall skeleton (total body) also improved with treatment. Of patients who had side effects after receiving setrusumab, most were mild or moderate intensity. Overall, setrusumab improved the bones of patients with OI with no serious safety concerns. More studies will include even more patients to see how setrusumab can improve their bones.
© The Author(s) 2024. Published by Oxford University Press on behalf of the American Society for Bone and Mineral Research.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

