Brain inflammation co-localizes highly with tau in mild cognitive impairment due to early-onset Alzheimer's disease
- PMID: 39013020
- PMCID: PMC11706285
- DOI: 10.1093/brain/awae234
Brain inflammation co-localizes highly with tau in mild cognitive impairment due to early-onset Alzheimer's disease
Abstract
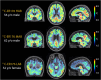
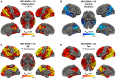
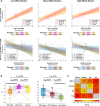
Brain inflammation, with an increased density of microglia and macrophages, is an important component of Alzheimer's disease and a potential therapeutic target. However, it is incompletely characterized, particularly in patients whose disease begins before the age of 65 years and, thus, have few co-pathologies. Inflammation has been usefully imaged with translocator protein (TSPO) PET, but most inflammation PET tracers cannot image subjects with a low-binder TSPO rs6971 genotype. In an important development, participants with any TSPO genotype can be imaged with a novel tracer, 11C-ER176, that has a high binding potential and a more favourable metabolite profile than other TSPO tracers currently available. We applied 11C-ER176 to detect brain inflammation in mild cognitive impairment (MCI) caused by early-onset Alzheimer's disease. Furthermore, we sought to correlate the brain localization of inflammation, volume loss, elevated amyloid-β (Aβ)and tau. We studied brain inflammation in 25 patients with early-onset amnestic MCI (average age 59 ± 4.5 years, 10 female) and 23 healthy controls (average age 65 ± 6.0 years, 12 female), both groups with a similar proportion of all three TSPO-binding affinities. 11C-ER176 total distribution volume (VT), obtained with an arterial input function, was compared across patients and controls using voxel-wise and region-wise analyses. In addition to inflammation PET, most MCI patients had Aβ (n = 23) and tau PET (n = 21). For Aβ and tau tracers, standard uptake value ratios were calculated using cerebellar grey matter as region of reference. Regional correlations among the three tracers were determined. Data were corrected for partial volume effect. Cognitive performance was studied with standard neuropsychological tools. In MCI caused by early-onset Alzheimer's disease, there was inflammation in the default network, reaching statistical significance in precuneus and lateral temporal and parietal association cortex bilaterally, and in the right amygdala. Topographically, inflammation co-localized most strongly with tau (r = 0.63 ± 0.24). This correlation was higher than the co-localization of Aβ with tau (r = 0.55 ± 0.25) and of inflammation with Aβ (0.43 ± 0.22). Inflammation co-localized least with atrophy (-0.29 ± 0.26). These regional correlations could be detected in participants with any of the three rs6971 TSPO polymorphisms. Inflammation in Alzheimer's disease-related regions correlated with impaired cognitive scores. Our data highlight the importance of inflammation, a potential therapeutic target, in the Alzheimer's disease process. Furthermore, they support the notion that, as shown in experimental tissue and animal models, the propagation of tau in humans is associated with brain inflammation.
Keywords: 11C-ER176 PET; TSPO; early-onset Alzheimer’s disease; inflammation; mild cognitive impairment.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
J.C.M. received research funding from Eli Lilly, parent company of Avid Radiopharmaceuticals, manufacturer of flortaucipir. The rest of the authors declare no competing interests.
Figures