mRNA-Engineered CD5-CAR-γδTCD5- Cells for the Immunotherapy of T-Cell Acute Lymphoblastic Leukemia
- PMID: 39013083
- PMCID: PMC11425277
- DOI: 10.1002/advs.202400024
mRNA-Engineered CD5-CAR-γδTCD5- Cells for the Immunotherapy of T-Cell Acute Lymphoblastic Leukemia
Abstract
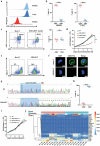
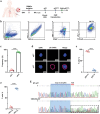
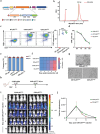
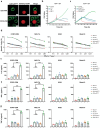
Clinical trials of Chimeric Antigen Receptor T-cell (CAR-T) therapy have demonstrated remarkable success in treating both solid tumors and hematological malignancies. Nanobodies (Nbs) have emerged as promising antigen-targeting domains for CARs, owing to their high specificity, robust stability, and strong affinity, leading to significant advancements in the field of Nb-CAR-T. In the realm of T-cell acute lymphoblastic leukemia (T-ALL) targets, CD5 stands out as a potentially excellent candidate for T-cell-based CAR therapy, due to its distinct expression on the surface of malignant T-ALL cells. To mitigate graft-versus-host disease associated with allogeneic CAR-T, γδT cells are selected and stimulated from peripheral blood mononuclear cells, and γδT cells are engineered via CRISPR/Cas9 to eliminate fratricide, enabling the creation of fratricide-resistant CAR-γδTCD5- cells. In vitro transcribed (IVT) mRNA is used to construct CAR-T, presenting a safer, faster, and cost-effective method compared to traditional viral vector approaches. In this study, a CD5-VHH library is constructed, and specific CD5-nanobodies are screened for subsequent use in CD5-CAR-γδTCD5- therapy. IVT-mRNA-CD5-CAR-γδTCD5- cells exhibited favorable functional characteristics and demonstrated antitumor efficacy against malignant T cell lines, underlining the potential for advancing mRNA-CD5-CAR-γδTCD5- therapy.
Keywords: CAR‐T; IVT‐mRNA; T‐ALL; γδT cells.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- June C. H., O'Connor R. S., Kawalekar O. U., Ghassemi S., Milone M. C., Science 2018, 359, 1361. - PubMed
-
- Hamers‐Casterman C., Atarhouch T., Muyldermans S., Robinson G., Hamers C., Songa E. B., Bendahman N., Hamers R., Nature 1993, 363, 446. - PubMed
-
- Harmsen M. M., Ruuls R. C., Nijman I. J., Niewold T. A., Frenken L. G., de Geus B., Mol. Immunol. 2000, 37, 579. - PubMed
-
- Xin Yu J., Hubbard‐Lucey V. M., Tang J., Nat. Rev. Drug Discov. 2019, 18, 821. - PubMed
MeSH terms
Substances
Grants and funding
- 82073404/National Natural Science Foundation of China
- ZYJC21003/Sichuan University
- 2023YFC3403303/National Key Research and Development Program of China
- TFJC2023010006/Frontiers Medical Center, Tianfu Jincheng Laboratory Foundation
- 2022CJE09013/Major scientific and technological achievements transformation project, Ningxia Hui Autonomous Region
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
