Association of CSF α-Synuclein Seeding Amplification Assay Results With Clinical Features of Possible and Probable Dementia With Lewy Bodies
- PMID: 39013126
- PMCID: PMC11238940
- DOI: 10.1212/WNL.0000000000209656
Association of CSF α-Synuclein Seeding Amplification Assay Results With Clinical Features of Possible and Probable Dementia With Lewy Bodies
Abstract
Background and objectives: The clinical diagnosis of dementia with Lewy bodies (DLB) depends on identifying significant cognitive decline accompanied by core features of parkinsonism, visual hallucinations, cognitive fluctuations, and REM sleep behavior disorder (RBD). Hyposmia is one of the several supportive features. α-Synuclein seeding amplification assays (αSyn-SAAs) may enhance diagnostic accuracy by detecting pathologic αSyn seeds in CSF. In this study, we examine how different clinical features associate with CSF αSyn-SAA positivity in a large group of clinically diagnosed participants with DLB.
Methods: Cross-sectional and longitudinal CSF samples from the multicentered observational cohort study of the DLB Consortium and similar studies within the Parkinson's Disease Biomarker Program, contributed by academic medical centers in the United States, underwent αSyn-SAA testing. Participants included those clinically diagnosed with DLB and 2 control cohorts. Associations between core DLB features and olfaction with αSyn-SAA positivity were evaluated using logistic regression.
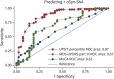
Results: CSF samples from 191 participants diagnosed with DLB (mean age 69.9 ± 6.8, 15% female), 50 age-matched and sex-matched clinical control participants, and 49 younger analytical control participants were analyzed. Seventy-two percent (137/191) of participants with DLB had positive αSyn-SAAs vs 4% of the control groups. Among participants with DLB, those who were αSyn-SAA-positive had lower Montreal Cognitive Assessment scores (18.8 ± 5.7 vs 21.2 ± 5.2, p = 0.01), had worse parkinsonism on the Movement Disorders Society Unified Parkinson's Disease Rating Scale part III (33.8 ± 15.1 vs 25.6 ± 16.4, p = 0.001), were more likely to report RBD (114/133 [86%] vs 33/53 [62%], p < 0.0001), and had worse hyposmia on the University of Pennsylvania Smell Identification Test (UPSIT) (94/105 [90%] below 15th percentile vs 14/44 [32%], p < 0.0001). UPSIT percentile had the highest area under the curve (0.87, 95% CI 0.81-0.94) in predicting αSyn-SAA positivity and participants scoring at or below the 15th percentile of age and sex normative values had 18.3 times higher odds (95% CI 7.52-44.6) of having a positive αSyn-SAA test. Among 82 participants with longitudinal CSF samples, 81 (99%) had the same αSyn-SAA result for initial and follow-up specimens.
Discussion: A substantial proportion of clinically diagnosed participants with DLB had negative αSyn-SAA results. Hyposmia was the strongest clinical predictor of αSyn-SAA positivity. Hyposmia and αSyn-SAA may have utility in improving the diagnostic assessment of individuals with potential DLB.
Classification of evidence: This study provided Class III evidence that CSF αSyn-SAA distinguishes patients with clinically diagnosed DLB from normal controls.
Conflict of interest statement
J.B. Leverenz receives research funding from GE Healthcare and the Lewy Body Dementia Association. A.S. Taylor is an employee of the Lewy Body Dementia Association. K.R. MacLeod is an employee of Amprion Inc. J.S. Middleton is an employee of Amprion Inc. J.E. Galvin reports a consulting agreement with GE Healthcare. D. Galasko reports a consulting agreement with GE Healthcare. A.C. Bozoki has been a site investigator for dementia with Lewy bodies trials sponsored by Cognition Therapeutics and EIP. J.E. Fleisher has been a site investigator for dementia with Lewy bodies trials sponsored by Cognition Therapeutics and EIP. L.S. Honig receives services in kind (research assays) from Amprion, Inc. The other authors report no relevant disclosures. Go to
Figures