Effect of Adding a Six-Week Course of Doxycycline to Intensive Hygiene-Based Care for Improving Lymphedema in a Rural Setting of Mali: A Double-Blind, Randomized Controlled 24-Month Trial
- PMID: 39013374
- PMCID: PMC11448486
- DOI: 10.4269/ajtmh.23-0908
Effect of Adding a Six-Week Course of Doxycycline to Intensive Hygiene-Based Care for Improving Lymphedema in a Rural Setting of Mali: A Double-Blind, Randomized Controlled 24-Month Trial
Abstract
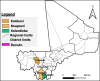
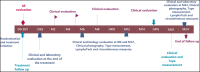
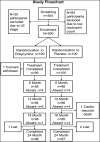
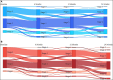
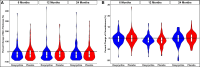
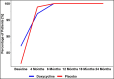
Lymphedema (LE) is one the most disfiguring chronic manifestations of lymphatic filariasis. Its management relies primarily on limb hygiene and local care. A previous study in Ghana demonstrating a beneficial effect of doxycycline on LE led to the current multicenter trial on the efficacy of doxycycline in filarial LE. A randomized placebo-controlled trial was initiated in two rural health districts in Mali. Patients with LE stages 1-3 were randomized to receive either doxycycline (200 mg/day) or placebo over a 6-week monitored treatment period and were then followed every 6 months for 2 years. Both groups received materials for limb hygiene that was carried out daily for the entire 2-year study. The primary endpoint was lack of progression in LE stage at 24 months. One hundred patients were enrolled in each study arm. The baseline sociodemographic characteristics of each group were largely similar. There was no significant difference at month 24 after treatment initiation in the number of subjects showing progression in LE stage between the two treatment arms (P = 0.5921). Importantly, however, the number of attacks of acute adenolymphangitis (ADLA) was reduced in both arms, but there was no significant difference between the two groups at any follow-up time point (all P >0.23). Doxycycline was well tolerated in those receiving the drug. When added to daily self-administered limb hygiene, a 6-week course of doxycycline (200 mg) was not superior to placebo in increasing the improvement associated with hygiene alone in LE volume, stage, or frequency of ADLA attacks over a 24-month period.
Conflict of interest statement
Disclosures: The protocol was approved by the Western Institutional Review Board (Olympia, WA) and additionally by the University of Sciences, Techniques and Technologies of Bamako’s local ethics committee (No2016/150/CE/FMPOS). Approval from the Ministry of Health was obtained for study drug importation and for trial implementation. Informed written consent was obtained from all individuals. Participants who could not read and understand French were assisted by a family member or another trusted person who could read and understand French. The role of this person was to read the information sheet and consent form and explain them to the participant. This person was also asked to sign the consent form as a witness. The participant’s fingerprint was placed on the consent form as his/her signature.
Authors’ contributions: Y. I. Coulibaly, A. F. Diabate, M. Sangare, E. A. Ottesen, A. Hoerauf, U. Klarmann-Schulz, S. Y. Coulibaly, S. S. Doumbia, M. Stephens, J. Horton, C. Mackenzie, and T. B. Nutman designed and conceived the study; Y. I. Coulibaly, E. A. Ottesen, C. Mackenzie, A. Hoerauf, U. Klarmann-Schulz, S. Sullivan, and T. B. Nutman approved the final version of the manuscript and helped with the analysis and drafting of the manuscript; Y. I. Coulibaly, A. F. Diabate, M. Sangare, S. O Thera, D. Tanapo, M. Stephens, H. Dolo, M. E. Coulibaly, L. Soumaoro, A. Zeguime, A. Diarra, U. Klarmann-Schulz, S. Sullivan, A. Majewski, and Y. Sanogo collected and processed the samples and drafted the manuscript; Y. I. Coulibaly, A. F. Diabate, M. Sangare, S. O Thera, D. Tanapo, M. Stephens, H. Dolo, M. E. Coulibaly, L. Soumaoro, U. Klarmann-Schulz, S. Sullivan, and Y. Sanogo managed the data, did the statistical analysis, and helped to draft the manuscript. All authors read and approved the final version of the manuscript.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

