Study protocol for a prospective, multicentre study of hypercortisolism in patients with difficult-to-control type 2 diabetes (CATALYST): prevalence and treatment with mifepristone
- PMID: 39013654
- PMCID: PMC11253743
- DOI: 10.1136/bmjopen-2023-081121
Study protocol for a prospective, multicentre study of hypercortisolism in patients with difficult-to-control type 2 diabetes (CATALYST): prevalence and treatment with mifepristone
Abstract
Introduction:
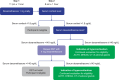
Even with recent treatment advances, type 2 diabetes (T2D) remains poorly controlled for many patients, despite the best efforts to adhere to therapies and lifestyle modifications. Although estimates vary, studies indicate that in >10% of individuals with difficult-to-control T2D, hypercortisolism may be an underlying contributing cause. To better understand the prevalence of hypercortisolism and the impact of its treatment on T2D and associated comorbidities, we describe the two-part Hyper
Methods and analysis: In part 1, approximately 1000 participants with difficult-to-control T2D (haemoglobin A1c (HbA1c) 7.5%-11.5% despite multiple therapies) are screened with a 1 mg dexamethasone suppression test (DST). Those with post-DST cortisol >1.8 µg/dL and dexamethasone level ≥140 ng/dL are identified to have hypercortisolism (part 1 primary endpoint), have morning adrenocorticotropic hormone (ACTH) and dehydroepiandrosterone sulfate (DHEAS) measured and undergo a non-contrast adrenal CT scan. Those requiring evaluation for elevated ACTH are referred for care outside the study; those with ACTH and DHEAS in the range may advance to part 2, a randomised, double-blind, placebo-controlled trial to evaluate the impact of treating hypercortisolism with the competitive glucocorticoid receptor antagonist mifepristone (Korlym®). Participants are randomised 2:1 to mifepristone or placebo for 24 weeks, stratified by the presence/absence of an abnormal adrenal CT scan. Mifepristone is dosed at 300 mg once daily for 4 weeks, then 600 mg daily based on tolerability and clinical improvement, with an option to increase to 900 mg. The primary endpoint of part 2 assesses changes in HbA1c in participants with hypercortisolism with or without abnormal adrenal CT scan. Secondary endpoints include changes in antidiabetes medications, cortisol-related comorbidities and quality of life.
Ethics and dissemination: The study has been approved by Cleveland Clinic IRB (Cleveland, Ohio, USA) and Advarra IRB (Columbia, Maryland, USA). Findings will be presented at scientific meetings and published in peer-reviewed journals.
Trial registration number: NCT05772169.
Keywords: Adrenal disorders; Clinical Trial; DIABETES & ENDOCRINOLOGY; Endocrine tumours; General diabetes; General endocrinology.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RAF reports: advisory board with AstraZeneca, Novo Nordisk, Boehringer Ingelheim, Intarcia, Renalytix, Corcept Therapeutics; research support from Boehringer Ingelheim, AstraZeneca; speaker's bureau with AstraZeneca. RJA reports: research support from Neurocrine Biosciences and Diurnal, Spruce Biosciences, Corcept Therapeutics, Sparrow Pharmaceuticals; consulting fees from Quest Diagnostics, Corcept Therapeutics, Xeris Pharmaceuticals, Crinetics Pharmaceuticals, Adrenas Therapeutics, PhaseBio Pharmaceuticals, Novo Nordisk, Recordati Rare Diseases, H Lundbeck A/S, OMass Therapeutics, Sparrow Pharmaceuticals. IB reports: consulting and/or advisory board with Corcept, HRA Pharma, Recordati, Sparrow, Neurocrine, Diurnal, Spruce; data safety monitoring board with Adrenas; funding for investigator initiated award from Recordati. LB reports: contracted fees paid to the Ochsner Clinic Foundation by Novo Nordisk for consulting work, and to Corcept Therapeutics for both speaking and consulting services. RSB reports: grant funding from Corcept pharmaceuticals; research funding from Novo, Lilly and Novartis; and speaker bureaus for Novo, Lilly, AstraZeneca, Amgen,and Bayer. JBB reports: contracted fees and travel support for contracted activities for consulting work paid to the University of North Carolina by Novo Nordisk; grant support from Dexcom, Insulet, NovaTarg, Novo Nordisk, Sanofi, Tolerion and vTv Therapeutics; personal compensation for consultation from Alkahest, Altimmune, Anji, AstraZeneca, Bayer, Biomea Fusion, Boehringer Ingelheim, CeQur, Cirius Therapeutics, Corcept Therapeutics, Eli Lilly, Fortress Biotech, GentiBio, Glycadia, Glyscend, Janssen, MannKind, Medtronic, Mellitus Health, Moderna, Pendulum Therapeutics, Praetego, Sanofi, Stability Health, Terns, Valo and Zealand Pharma; stock/options in Glyscend, Mellitus Health, Pendulum Therapeutics, PhaseBio, Praetego, Stability Health. JWF reports: consultant and investigator for Corcept, Recordati. VAF reports: research support (to Tulane): grants from Fractyl, Jaguar Gene Therapy, Corcept; honoraria for consulting and lectures from Asahi, Bayer, Abbott, Boehringer Ingelheim, Corcept; stock options in Mellitus Health, BRAVO4Health; stock in Amgen, Abbott; patents pending for 1) BRAVO risk engine for predicting diabetes complications, 2) PAX4 gene therapy for type 1 diabetes. JPF reports: research support from Akero, Altimmune, Boehringer Ingelheim, 89bio, Eli Lilly, Intercept, IONIS, Janssen, Madrigal, Merck, NorthSea Therapeutics, Novartis, Novo Nordisk, Oramed, Pfizer, Sanofi, Shionogi, Corcept Therapeutics; advisory boards and consulting with Akero, Altimmune, Boehringer Ingelheim, Carmot Therapeutics, Echosens, 89bio, Eli Lilly, Gilead, Intercept, Merck, Novo Nordisk, Pfizer, Sanofi, Sun Pharma; speaker’s bureau with Eli Lilly; employment and stock ownership in Biomea Fusion. OH reports: scientific advisory board participation with Corcept Therapeutics, Strongbridge Pharma, Recordati Rare Diseases, Amryt Pharma, Neurocrine Biosciences, Lantheus, Xeris. YH reports: research grant from Amgen, Applied Therapeutic, Corcept, Ionis, Lilly, Merck, Regor; advisory/consultant role with Amgen, Applied Therapeutic, AZ, Bayer, BI, Corcept, Esperion, Mankind Pharma, Merck, Merck-Pfizer, Novartis, Novo Nordisk, Sanofi; speaker’s bureau with Bayer, Esperion, Novo Nordisk. REP reports: consulting fees from Bayer AG, Corcept Therapeutics Incorporated, Dexcom, Hanmi Pharmaceutical Co., Merck, Novo Nordisk, Pfizer, Sanofi, Scohia Pharma, Sun Pharmaceutical Industries; grants/research support from Hanmi Pharmaceutical Co., Janssen, Metavention, Novo Nordisk, Poxel SA, Sanofi. All funds are paid directly to REP’s employer, AdventHealth, a non-profit organisation that supports education and research. JR reports: advisory panels with Applied Therapeutics, Boehringer Ingelheim, Eli Lilly and Company, Hanmi, Intarcia, Novo Nordisk, Oramed, Sanofi, Scholar Rock, Structure Therapeutics, Terns Pharma and Zealand; research support from Applied Therapeutics, AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Novartis, Intarcia, Merck, Novo Nordisk, Oramed, Pfizer, Sanofi. ICT, AGM and DE report: employed by and own stock in Corcept Therapeutics.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
