Association of circulating tumor DNA with patient prognosis in surgically resected renal cell carcinoma
- PMID: 39013784
- PMCID: PMC11449105
- DOI: 10.1093/oncolo/oyae180
Association of circulating tumor DNA with patient prognosis in surgically resected renal cell carcinoma
Abstract
Background: Despite complete resection, 20%-50% of patients with localized renal cell carcinoma (RCC) experience recurrence within 5 years. Accurate assessment of prognosis in high-risk patients would aid in improving outcomes. Here we evaluate the use of circulating tumor DNA (ctDNA) in RCC using banked samples and clinical data from a single institution.
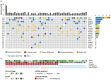
Methods: The cohort consisted of 45 RCC patients (≥pT1b) who underwent complete resection. The presence of ctDNA in plasma was determined using a personalized, tumor-informed ctDNA assay (Signatera RUO, Natera, Inc.). Relationships with outcomes and other relevant clinical variables were assessed. The median follow-up was 62 months.
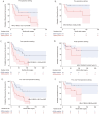
Results: Plasma ctDNA was detected in 18 out of 36 patients (50%) pre-operatively and was associated with increased tumor size (mean 9.3 cm vs. 7.0 cm, P < .05) and high Fuhrman grade (60% grades III-IV vs 27% grade II, P = .07). The presence of ctDNA, either pre-operatively or at any time post-operatively, was associated with inferior relapse-free survival (HR = 2.70, P = .046; HR = 3.23, P = .003, respectively). Among patients who were ctDNA positive at any time point, the sensitivity of relapse prediction was 84% with a PPV of 90%. Of note, ctDNA positivity at a post-surgical time point revealed a PPV of 100% and NPV of 64%. The lack of ctDNA detection at both time points yielded an NPV of 80%.
Conclusions: Detection of plasma ctDNA using a personalized assay is prognostic of recurrence in patients with resected RCC. Herein, we describe a successful approach for its application and identify potential limitations to be addressed in future studies.
Keywords: circulating tumor DNA; liquid biopsy; recurrence; renal cell carcinoma.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
The following authors are employees of Natera, Inc. (Ekaterina Kalashnikova, Hsin-Ta Wu, Sumedha Sudhaman, Himanshu Sethi, Mustafa Balcioglu, Adam ElNaggar, Minetta Liu, and Alexey Aleshin) and own stock, or options to stock, in the company. Author P.H.A.: Natera (research support), Janssen (consultant, research support), NCI (research support), DoD (research support). Author M.C.L reports Grants/Contracts: Funding to Institution (Mayo) from: Eisai, Exact Sciences, Genentech, Genomic Health, GRAIL, Menarini Silicon Biosystems, Merck, Novartis, Seattle Genetics, Tesaro; Travel Support Reimbursement from AstraZeneca, Genomic Health, Ionis; Ad hoc advisory board meetings. All funds to Mayo Clinic. No personal compensation from: AstraZeneca, Celgene, Roche/Genentech, Genomic Health, GRAIL, Ionis, Merck, Pfizer, Seattle Genetics, Syndax.
Figures

Comment in
-
Liquid biopsy in renal cell carcinoma.Oncologist. 2024 Oct 3;29(10):821-823. doi: 10.1093/oncolo/oyae230. Oncologist. 2024. PMID: 39187381 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

