Aging aggravates aortic aneurysm and dissection via miR-1204-MYLK signaling axis in mice
- PMID: 39013850
- PMCID: PMC11252124
- DOI: 10.1038/s41467-024-50036-2
Aging aggravates aortic aneurysm and dissection via miR-1204-MYLK signaling axis in mice
Abstract
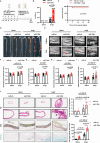
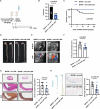
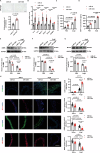
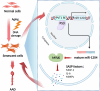
The mechanism by which aging induces aortic aneurysm and dissection (AAD) remains unclear. A total of 430 participants were recruited for the screening of differentially expressed plasma microRNAs (miRNAs). We found that miR-1204 is significantly increased in both the plasma and aorta of elder patients with AAD and is positively correlated with age. Cell senescence induces the expression of miR-1204 through p53 interaction with plasmacytoma variant translocation 1, and miR-1204 induces vascular smooth muscle cell (VSMC) senescence to form a positive feedback loop. Furthermore, miR-1204 aggravates angiotensin II-induced AAD formation, and inhibition of miR-1204 attenuates β-aminopropionitrile monofumarate-induced AAD development in mice. Mechanistically, miR-1204 directly targets myosin light chain kinase (MYLK), leading to the acquisition of a senescence-associated secretory phenotype (SASP) by VSMCs and loss of their contractile phenotype. MYLK overexpression reverses miR-1204-induced VSMC senescence, SASP and contractile phenotypic changes, and the decrease of transforming growth factor-β signaling pathway. Our findings suggest that aging aggravates AAD via the miR-1204-MYLK signaling axis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Isselbacher EM, et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022;146:E334–E482. doi: 10.1161/CIR.0000000000001106. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

