Neoadjuvant and adjuvant pembrolizumab in advanced high-grade serous carcinoma: the randomized phase II NeoPembrOV clinical trial
- PMID: 39013870
- PMCID: PMC11252284
- DOI: 10.1038/s41467-024-46999-x
Neoadjuvant and adjuvant pembrolizumab in advanced high-grade serous carcinoma: the randomized phase II NeoPembrOV clinical trial
Abstract
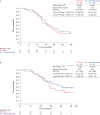
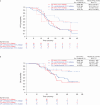
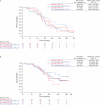
This open-label, non-comparative, 2:1 randomized, phase II trial (NCT03275506) in women with stage IIIC/IV high-grade serous carcinoma (HGSC) for whom upfront complete resection was unachievable assessed whether adding pembrolizumab (200 mg every 3 weeks) to standard-of-care carboplatin plus paclitaxel yielded a complete resection rate (CRR) of at least 50%. Postoperatively patients continued assigned treatment for a maximum of 2 years. Postoperative bevacizumab was optional. The primary endpoint was independently assessed CRR at interval debulking surgery. Secondary endpoints were Completeness of Cytoreduction Index (CCI) and peritoneal cancer index (PCI) scores, objective and best response rates, progression-free survival, overall survival, safety, postoperative morbidity, and pathological complete response. The CRR in 61 pembrolizumab-treated patients was 74% (one-sided 95% CI = 63%), exceeding the prespecified ≥50% threshold and meeting the primary objective. The CRR without pembrolizumab was 70% (one-sided 95% CI = 54%). In the remaining patients CCI scores were ≥3 in 27% of the standard-of-care group and 18% of the investigational group and CC1 in 3% of the investigational group. PCI score decreased by a mean of 9.6 in the standard-of-care group and 10.2 in the investigational group. Objective response rates were 60% and 72%, respectively, and best overall response rates were 83% and 90%, respectively. Progression-free survival was similar with the two regimens (median 20.8 versus 19.4 months in the standard-of-care versus investigational arms, respectively) but overall survival favored pembrolizumab-containing therapy (median 35.3 versus 49.8 months, respectively). The most common grade ≥3 adverse events with pembrolizumab-containing therapy were anemia during neoadjuvant therapy and infection/fever postoperatively. Pembrolizumab was discontinued prematurely because of adverse events in 23% of pembrolizumab-treated patients. Combining pembrolizumab with neoadjuvant chemotherapy is feasible for HGSC considered not completely resectable; observed activity in some subgroups justifies further evaluation to improve understanding of the role of immunotherapy in HGSC.
© 2024. The Author(s).
Conflict of interest statement
I.L.R.-C. reports personal honoraria from Agenus, Blueprint, BMS, PharmaMar, Genmab, Pfizer, AstraZeneca, Roche, GSK, MSD, Deciphera, Mersana, Merck Sereno, Novartis, Amgen, MacroGenics, Tesaro, and Clovis; honoraria to her institution from GSK, MSD, Roche, and BMS; consulting/advisory roles for AbbVie, Agenus, Advaxis, BMS, PharmaMar, Genmab, Pfizer, AstraZeneca, Roche/Genentech, GSK, MSD, Deciphera, Mersana, Merck Sereno, Novartis, Amgen, Tesaro and Clovis; research grant/funding from MSD, Roche and BMS (self) and MSD, Roche, BMS, Novartis, AstraZeneca and Merck Sereno (to institution); and travel support from Roche, AstraZeneca, and GSK. E.K. reports honoraria from AstraZeneca, Roche, Sanofi, Tesaro, and GSK; consulting/advisory roles for AstraZeneca, Roche, Sanofi, Tesaro and GSK; speakers’ bureau for AstraZeneca, Roche, Sanofi, Tesaro, and GSK; and research funding from AstraZeneca, Roche, Sanofi, Tesaro and GSK. M.L. reports consulting/advisory roles for Pierre Fabre, GSK, and Daiichi; research funding from Veracyte; and travel/accommodation/expenses from Mundi Pharma, Pfizer, GSK, and MSD. L.B.L. reports travel/accommodation/expenses from Servier. F.P. reports consulting/advisory role for AstraZeneca. F.S. reports honoraria from AstraZeneca, Clovis Oncology, MSD, GSK-Tesaro, and Sandoz (Novartis); consulting/advisory roles for AstraZeneca, GSK-Tesaro and MSD; speakers’ bureau for AstraZeneca, MSD, and GSK-Tesaro; and travel/accommodation/expenses from Roche, AstraZeneca, MSD, and GSK-Tesaro. E.C. reports the following, all for an immediate family member: employment with Sanofi; consulting/advisory role for MSD, BMS, Ipsen, and AstraZeneca; speakers’ bureau for BMS, Ipsen, AstraZeneca, and Janssen; research funding from Pfizer; and travel/accommodation/expenses from Janssen. O.L.S. reports honoraria from MSD and Clovis, and travel/accommodation/expenses from Eisai. A.A. reports honoraria from GlaxoSmithKline. F.J. reports honoraria from Clovis, Amgen, GSK, Ipsen, BMS, MSD, AstraZeneca, Astellas, Janssen and Bayer; consultancy from AstraZeneca, GSK, Janssen and Ipsen; travel/accommodation/expenses from Ipsen and GSK; participation on a data safety monitoring/advisory board for GSK; and participation on the GINECO guidelines committee. O.T. reports honoraria from Roche, MSD/Merck, Novartis/Sandoz, Pfizer, Lilly, AstraZeneca, Daiichi Sankyo, Eisai, Pierre Fabre, Seagen, and Gilead, and research funding from Roche, MSD/Merck and BMS. The remaining authors declare no competing interests.
Figures
References
-
- Vergote I, et al. EORTC; MRC CHORUS study investigators. Neoadjuvant chemotherapy versus debulking surgery in advanced tubo-ovarian cancers: pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncol. 2018;19:1680–1687. doi: 10.1016/S1470-2045(18)30566-7. - DOI - PubMed
-
- Fagotti A, et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): final analysis of peri-operative outcome. Eur. J. Cancer. 2016;59:22–33. doi: 10.1016/j.ejca.2016.01.017. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

