SGLT2 inhibitors attenuate endothelial to mesenchymal transition and cardiac fibroblast activation
- PMID: 39013942
- PMCID: PMC11252266
- DOI: 10.1038/s41598-024-65410-9
SGLT2 inhibitors attenuate endothelial to mesenchymal transition and cardiac fibroblast activation
Abstract
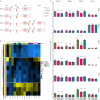
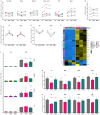
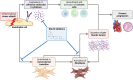
Beneficial effects of sodium glucose co-transporter 2 inhibitors (SGLT2is) in cardiovascular diseases have been extensively reported leading to the inclusion of these drugs in the treatment guidelines for heart failure. However, molecular actions especially on non-myocyte cells remain uncertain. We observed dose-dependent inhibitory effects of two SGLT2is, dapagliflozin (DAPA) and empagliflozin (EMPA), on inflammatory signaling in human umbilical vein endothelial cells. Proteomic analyses and subsequent enrichment analyses discovered profound effects of these SGLT2is on proteins involved in mitochondrial respiration and actin cytoskeleton. Validation in functional oxygen consumption measurements as well as tube formation and migration assays revealed strong impacts of DAPA. Considering that most influenced parameters played central roles in endothelial to mesenchymal transition (EndMT), we performed in vitro EndMT assays and identified substantial reduction of mesenchymal and fibrosis marker expression as well as changes in cellular morphology upon treatment with SGLT2is. In line, human cardiac fibroblasts exposed to DAPA showed less proliferation, reduced ATP production, and decelerated migration capacity while less extensive impacts were observed upon EMPA. Mechanistically, sodium proton exchanger 1 (NHE1) as well as sodium-myoinositol cotransporter (SMIT) and sodium-multivitamin cotransporter (SMVT) could be identified as relevant targets of SGLT2is in non-myocyte cardiovascular cells as validated by individual siRNA-knockdown experiments. In summary, we found comprehensive beneficial effects of SGLT2is on human endothelial cells and cardiac fibroblasts. The results of this study therefore support a distinct effect of selected SGLT2i on non-myocyte cardiovascular cells and grant further insights into potential molecular mode of action of these drugs.
Keywords: Cardiovascular diseases; Cell migration; Endothelial cell; Fibroblasts; Inflammation; Oxygen consumption; Sodium-glucose transporter 2 inhibitors.
© 2024. The Author(s).
Conflict of interest statement
T.T. is founder and CSO/CMO of Cardior Pharmaceuticals GmbH, a wholly-owned subsidiary of Novo Nordisk A/S (outside of the content of this manuscript). All other authors have no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

