Metabolic imaging across scales reveals distinct prostate cancer phenotypes
- PMID: 39013948
- PMCID: PMC11252279
- DOI: 10.1038/s41467-024-50362-5
Metabolic imaging across scales reveals distinct prostate cancer phenotypes
Abstract
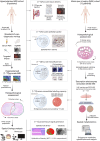
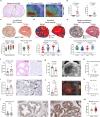
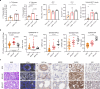
Hyperpolarised magnetic resonance imaging (HP-13C-MRI) has shown promise as a clinical tool for detecting and characterising prostate cancer. Here we use a range of spatially resolved histological techniques to identify the biological mechanisms underpinning differential [1-13C]lactate labelling between benign and malignant prostate, as well as in tumours containing cribriform and non-cribriform Gleason pattern 4 disease. Here we show that elevated hyperpolarised [1-13C]lactate signal in prostate cancer compared to the benign prostate is primarily driven by increased tumour epithelial cell density and vascularity, rather than differences in epithelial lactate concentration between tumour and normal. We also demonstrate that some tumours of the cribriform subtype may lack [1-13C]lactate labelling, which is explained by lower epithelial lactate dehydrogenase expression, higher mitochondrial pyruvate carrier density, and increased lipid abundance compared to lactate-rich non-cribriform lesions. These findings highlight the potential of combining spatial metabolic imaging tools across scales to identify clinically significant metabolic phenotypes in prostate cancer.
© 2024. The Author(s).
Conflict of interest statement
G.H., L.F., D.B., J.R., S.L., J.Y.T., S.T.B., and R.J.A.G. are AstraZeneca employees. F.A.G. has research support from GE Healthcare and AstraZeneca, grants from GlaxoSmithKline, and has consulted for AstraZeneca on behalf of the University of Cambridge. The remaining authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

