A systematic search for RNA structural switches across the human transcriptome
- PMID: 39014073
- PMCID: PMC11399106
- DOI: 10.1038/s41592-024-02335-1
A systematic search for RNA structural switches across the human transcriptome
Abstract
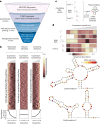
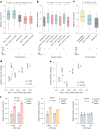
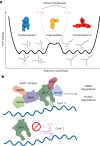
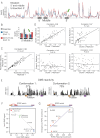
RNA structural switches are key regulators of gene expression in bacteria, but their characterization in Metazoa remains limited. Here, we present SwitchSeeker, a comprehensive computational and experimental approach for systematic identification of functional RNA structural switches. We applied SwitchSeeker to the human transcriptome and identified 245 putative RNA switches. To validate our approach, we characterized a previously unknown RNA switch in the 3' untranslated region of the RORC (RAR-related orphan receptor C) transcript. In vivo dimethyl sulfate (DMS) mutational profiling with sequencing (DMS-MaPseq), coupled with cryogenic electron microscopy, confirmed its existence as two alternative structural conformations. Furthermore, we used genome-scale CRISPR screens to identify trans factors that regulate gene expression through this RNA structural switch. We found that nonsense-mediated messenger RNA decay acts on this element in a conformation-specific manner. SwitchSeeker provides an unbiased, experimentally driven method for discovering RNA structural switches that shape the eukaryotic gene expression landscape.
© 2024. The Author(s).
Conflict of interest statement
M.K. and H.G. are inventors on a provisional patent related to this study. L.A.G. has filed patents on CRISPR functional genomics. The other authors have no competing interests.
Figures









References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

