Expression, characterization, and application of human-like recombinant gelatin
- PMID: 39014092
- PMCID: PMC11252100
- DOI: 10.1186/s40643-024-00785-1
Expression, characterization, and application of human-like recombinant gelatin
Abstract
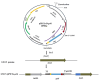
Gelatin is a product obtained through partial hydrolysis and thermal denaturation of collagen, belonging to natural biopeptides. With irreplaceable biological functions in the field of biomedical science and tissue engineering, it has been widely applied. The amino acid sequence of recombinant human-like gelatin was constructed through a newly designed hexamer composed of six protein monomer sequences in series, with the minimum repeating unit being the characteristic Gly-X-Y sequence found in type III human collagen α1 chain. The nucleotide sequence was subsequently inserted into the genome of Pichia pastoris to enable soluble secretion expression of recombinant gelatin. At the shake flask fermentation level, the yield of recombinant gelatin is up to 0.057 g/L, and its purity can rise up to 95% through affinity purification. It was confirmed in the molecular weight determination and amino acid analysis that the amino acid composition of the obtained recombinant gelatin is identical to that of the theoretically designed. Furthermore, scanning electron microscopy revealed that the freeze-dried recombinant gelatin hydrogel exhibited a porous structure. After culturing cells continuously within these gelatin microspheres for two days followed by fluorescence staining and observation through confocal laser scanning microscopy, it was observed that cells clustered together within the gelatin matrix, exhibiting three-dimensional growth characteristics while maintaining good viability. This research presents promising prospects for developing recombinant gelatin as a biomedical material.
Keywords: Pichia pastoris; Biomedical materials; Characterization; Monomer protein sequence; Recombinant human gelatin; Three- dimensional culture.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Betnér I, Földi P. New automated amino acid analysis by HPLC precolumns derivatization with fluorenylmethyloxycarbonylchloride. Chromatographia. 1986;22(7):381–387. doi: 10.1007/BF02268795. - DOI
-
- Butkowski RJ, Noelken ME, Hudson BG. Estimation of the size of collagenous proteins by electrophoresis and gel chromatography. Methods Enzymol. 1982;82C:410–423. doi: 10.1016/0076-6879(82)82076-4. - DOI
-
- Cherim M, Srbu R. Obtaining of collagen extracts used as biomaterials with applications in the Medical Field. J Euro Med Natur Sci. 2020;3(2):54–60.
Grants and funding
LinkOut - more resources
Full Text Sources

