Oxytocin treatment rescues irritability-like behavior in Cc2d1a conditional knockout mice
- PMID: 39014123
- PMCID: PMC11399130
- DOI: 10.1038/s41386-024-01920-4
Oxytocin treatment rescues irritability-like behavior in Cc2d1a conditional knockout mice
Abstract
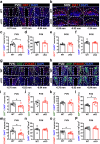
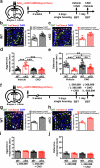
Irritability, a state of excessive reactivity to negative emotional stimuli, is common in individuals with autism spectrum disorder (ASD). Although it has a significant negative impact of patients' disease severity and quality of life, the neural mechanisms underlying irritability in ASD remain largely unclear. We have previously demonstrated that male mice lacking the Coiled-coil and C2 domain containing 1a (Cc2d1a) in forebrain excitatory neurons recapitulate numerous ASD-like behavioral phenotypes, including impaired social behaviors and pronounced repetitive behaviors. Here, using the bottle-brush test (BBT) to trigger and evaluate aggressive and defensive responses, we show that Cc2d1a deletion increases irritability-like behavior in male but not female mice, which is correlated with reduced number of oxytocin (OXT)-expressing neurons in the paraventricular nucleus (PVN) of the hypothalamus. Intranasal OXT administration or chemogenetic activation of OXT neurons in the PVN rescues irritability-like behavior in Cc2d1a conditional knockout (cKO) mice. Administration of a selective melanocortin receptor 4 agonist, RO27-3225, which potentiates endogenous OXT release, also alleviates irritability-like behavior in Cc2d1a cKO mice, an effect blocked by a specific OXT receptor antagonist, L-368,899. We additionally identify a projection connecting the posterior ventral segment of the medial amygdala (MeApv) and ventromedial nucleus of the ventromedial hypothalamus (VMHvl) for governing irritability-like behavior during the BBT. Chemogenetic suppression of the MeApv-VMHvl pathway alleviates irritability-like behavior in Cc2d1a cKO mice. Together, our study uncovers dysregulation of OXT system in irritability-like behavior in Cc2d1a cKO mice during the BBT and provide translatable insights into the development of OXT-based therapeutics for clinical interventions.
© 2024. The Author(s), under exclusive licence to American College of Neuropsychopharmacology.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

