Altered hypoxia-induced cellular responses and inflammatory profile in lung fibroblasts from COPD patients compared to control subjects
- PMID: 39014439
- PMCID: PMC11253402
- DOI: 10.1186/s12931-024-02907-x
Altered hypoxia-induced cellular responses and inflammatory profile in lung fibroblasts from COPD patients compared to control subjects
Abstract
Background: Chronic obstructive pulmonary disease (COPD) is a heterogeneous disease characterized by chronic bronchitis, emphysema and vascular remodelling. The disease is associated with hypoxia, inflammation and oxidative stress. Lung fibroblasts are important cells in remodelling processes in COPD, as main producers of extracellular matrix proteins but also in synthesis of growth factors and inflammatory mediators.
Methods: In this study we aimed to investigate if there are differences in how primary distal lung fibroblasts obtained from COPD patients and healthy subjects respond to hypoxia (1% O2) and pro-fibrotic stimuli with TGF-β1 (10 ng/mL). Genes and proteins associated with oxidative stress, endoplasmic reticulum stress, remodelling and inflammation were analysed with RT-qPCR and ELISA.
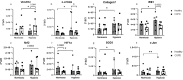
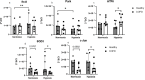
Results: Hypoxia induced differences in expression of genes involved in oxidative stress (SOD3 and HIF-1α), ER stress (IRE1, PARK and ATF6), apoptosis (c-Jun and Bcl2) and remodelling (5HTR2B, Collagen7 and VEGFR2) in lung fibroblasts from COPD subjects compared to control subjects, where COPD fibroblasts were in general less responsive. The release of VEGF-C was increased after hypoxia, whereas TGF-β significantly reduced the VEGF response to hypoxia and the release of HGF. COPD fibroblasts had a higher release of IL-6, IL-8, MCP-1 and PGE2 compared to lung fibroblasts from control subjects. The release of inflammatory mediators was less affected by hypoxia, whereas TGFβ1 induced differences in inflammatory profile between fibroblasts from COPD and control subjects.
Conclusion: These results suggest that there is an alteration of gene regulation of various stress responses and remodelling associated mediator release that is related to COPD and hypoxia, where fibroblasts from COPD patients have a deficient response.
Keywords: COPD; ER stress; Gene expression; Hypoxia; Inflammation; Lung fibroblasts; Oxidative stress; Remodelling.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

