Stereospecific Synthesis and Biological Evaluation of KRN7000 Analogues with Thio-modifications at the Acyl Moiety
- PMID: 39015265
- PMCID: PMC11247626
- DOI: 10.1021/acsmedchemlett.4c00199
Stereospecific Synthesis and Biological Evaluation of KRN7000 Analogues with Thio-modifications at the Acyl Moiety
Abstract
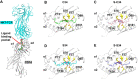
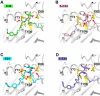
α-Galactosylceramide (KRN7000 or α-GalCer) analogues terminated with phenyl (Ph) groups at the acyl moiety possess more potency than KRN7000 to activate invariant natural killer T (iNKT) cells for inducing a T helper 1 (Th1)-biased immune response. However, biological activities of phenyl glycolipids with thio-modifications at the acyl moiety remain unknown, and facile approaches for highly stereoselective synthesis of KRN7000 and its analogues are rather scarce. Herein, we exploited 4,6-di-O-tert-butylsilylene (DTBS)-directed stereospecific galactosylation to efficiently synthesize various α-GalCer analogues bearing thioamide, terminal thiophenyl and dual modifications at the acyl moiety. Biological evaluations suggest that a new analogue S34 featuring a terminal Ph-S-Ph-F group exhibits a more superior Th1-biased immune response in mice. Molecular docking analysis revealed that the introduction of a sulfur atom influences vital hydrogen bonding interactions between glycolipids and the cluster of differentiation 1d (CDld), thus adjusting the stability of the glycolipid-CDld complex.
© 2024 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
The Role of Ribose Modifications on the Structural Stability of Nucleotide Analogues with α-Thiotriphosphate at the Active Site of SARS-CoV-2 RdRp.J Chem Inf Model. 2025 Jun 23;65(12):6102-6113. doi: 10.1021/acs.jcim.5c00152. Epub 2025 Jun 6. J Chem Inf Model. 2025. PMID: 40476830
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in adults and children aged 12 years and over.Health Technol Assess. 2008 May;12(19):iii-iv, 1-360. doi: 10.3310/hta12190. Health Technol Assess. 2008. PMID: 18485271
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
Cited by
-
Optimizing iNKT-driven immune responses against cancer by modulating CD1d in tumor and antigen presenting cells.Clin Immunol. 2024 Dec;269:110402. doi: 10.1016/j.clim.2024.110402. Epub 2024 Nov 17. Clin Immunol. 2024. PMID: 39561929 Free PMC article. Review.
-
Development of potential immunomodulatory ligands targeting natural killer T cells inspired by gut symbiont-derived glycolipids.Commun Chem. 2025 Apr 1;8(1):98. doi: 10.1038/s42004-025-01497-z. Commun Chem. 2025. PMID: 40169880 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

