B2R-Targeting Radiotracer for PET/MR Imaging of Hepatocellular Carcinoma and Guiding Anti-B2R Therapy
- PMID: 39015273
- PMCID: PMC11247633
- DOI: 10.1021/acsmedchemlett.4c00155
B2R-Targeting Radiotracer for PET/MR Imaging of Hepatocellular Carcinoma and Guiding Anti-B2R Therapy
Abstract
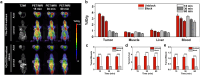
The bradykinin B2 receptor (B2R) is overexpressed in a wide variety of tumors and is a well-defined target for tumor imaging and therapy. The hybrid positron emission tomography/magnetic resonance imaging (PET/MRI) scanner is considered a noninvasive and advanced instrument for precise tumor imaging. In this work, we developed a novel B2R-targeting radiotracer, 68Ga-DOTA-icatibant, for quantifying B2R expression. 68Ga-DOTA-icatibant showed high stability, fast clearance and specific binding to B2R. PET/MR imaging revealed excellent tumor accumulation, and the uptake in tumors could be blocked by DOTA-icatibant. Icatibant-mediated anti-B2R therapy downregulated B2R expression in tumor cells and inhibited the growth of HepG2 tumors, and the decrease in tumor uptake was monitored by timely PET/MR imaging. Hematoxylin and eosin (H&E) and immunohistochemical staining results further demonstrated that the efficacy of anti-B2R could be accurately monitored with the developed PET/MR imaging radiotracer. 68Ga-DOTA-icatibant can be utilized to noninvasively determine B2R expression and dynamically and sensitively monitor the efficacy of anti-B2R therapy.
© 2024 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Ahn J. C.; Teng P. C.; Chen P. J.; Posadas E.; Tseng H. R.; Lu S. C.; Yang J. D. Detection of Circulating Tumor Cells and Their Implications as a Biomarker for Diagnosis, Prognostication, and Therapeutic Monitoring in Hepatocellular Carcinoma. Hepatology 2021, 73 (1), 422–436. 10.1002/hep.31165. - DOI - PMC - PubMed
-
- Finn R. S.; Ryoo B. Y.; Merle P.; Kudo M.; Bouattour M.; Lim H. Y.; Breder V.; Edeline J.; Chao Y.; Ogasawara S.; Yau T.; Garrido M.; Chan S. L.; Knox J.; Daniele B.; Ebbinghaus S. W.; Chen E.; Siegel A. B.; Zhu A. X.; Cheng A. L. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin Oncol 2020, 38 (3), 193–202. 10.1200/JCO.19.01307. - DOI - PubMed
LinkOut - more resources
Full Text Sources

