A facile one-pot synthesis of tetrahydrobenzo[ b]pyrans and 2-amino-4 H-chromenes under green conditions
- PMID: 39015477
- PMCID: PMC11249666
- DOI: 10.1039/d4ra04239e
A facile one-pot synthesis of tetrahydrobenzo[ b]pyrans and 2-amino-4 H-chromenes under green conditions
Abstract
This research developed a new nanocatalyst by incorporating nanocopper iodide onto the surface of a layered double hydroxides modified. This new nanocatalyst enables the green synthesis of tetrahydrobenzo[b]pyrans and 2-amino-4H-chromene derivatives through a one-pot, three-component reaction, demonstrating remarkable activity and selectivity. Key advantages of this method include increased products yield (86-96%), rapid reaction kinetics (5-23 minutes), low reaction temperature (40 °C), synthesis of new products, straightforward purification methods, catalyst recyclability (up to 4 cycles), and solvent-free conditions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing interests.
Figures

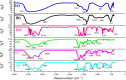




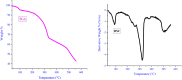
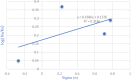


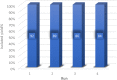
References
-
- Mondal S. Dasgupta S. Maji K. MgAl- Layered Double Hydroxide Nanoparticles for controlled release of Salicylate. Mater. Sci. Eng. C. 2016;68:557–564. - PubMed
-
- Khan S. Khan S. Asiri A. Toward the design of Zn–Al and Zn–Cr LDH wrapped in activated carbon for the solar assisted de-coloration of organic dyes. RSC Adv. 2016;6:83196–83208.
LinkOut - more resources
Full Text Sources

