Burosumab treatment of X-linked hypophosphatemia patients: interim analysis of the SUNFLOWER longitudinal, observational cohort study
- PMID: 39015507
- PMCID: PMC11250265
- DOI: 10.1093/jbmrpl/ziae079
Burosumab treatment of X-linked hypophosphatemia patients: interim analysis of the SUNFLOWER longitudinal, observational cohort study
Abstract
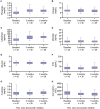
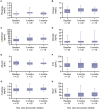
X-linked hypophosphatemia (XLH) is a genetic disease that results in excessive FGF23, chronic hypophosphatemia, and musculoskeletal abnormalities, with affected patients experiencing symptoms such as bone pain, bone deformity, fracture, and pseudofracture. Burosumab is a fully human monoclonal antibody that binds to FGF23, improving lowered serum 1,25(OH)2D and phosphate levels in patients with XLH. There are insufficient data on the use of burosumab, its safety, and the outcomes of treated patients in a real-world setting. The SUNFLOWER (Study of longitUdinal observatioN For patients with X-Linked hypOphosphatemic rickets/osteomalacia in collaboration With Asian partnERs) study is an ongoing longitudinal, observational cohort study of patients with XLH in Japan and South Korea. Enrollment occurred between April 2018 and December 2020. This interim analysis compared the background characteristics of patients who received burosumab with those who did not, and assessed improvements in biomarkers, physical and motor function, health-related quality-of-life (HRQOL) and other patient-reported outcome (PRO) measures, as well as the safety of burosumab treatment in 143 Japanese patients from 15 institutions over 6 mo. The patients had a median [interquartile range] age of 17.5 [11.0, 38.8] yr and 98 (68.5%) were female. Among patients aged <18 and ≥18 yr, 40/73 (54.8%) and 25/70 (35.7%) received burosumab, respectively. More patients aged ≥18 who received burosumab had bone pain at baseline vs those not treated with burosumab (6/25, 24.0% vs 2/45, 4.4%, p=.021). Patients treated with burosumab had improved serum phosphate and 1,25(OH)2D levels; moreover, rickets severity and HRQOL/PRO measures, such as pain, appeared to improve over 6 mo of burosumab treatment, and no new safety concerns were identified. This study identified trends in the background characteristics of patients with XLH who receive burosumab in real-world clinical practice. Furthermore, the results support the use of burosumab therapy in real-world settings.
Keywords: PTH/Vit D/FGF23; bone turnover markers; disorders of calcium/phosphate metabolism; osteomalacia; rickets.
© The Author(s) 2024. Published by Oxford University Press on behalf of the American Society for Bone and Mineral Research.
Conflict of interest statement
T.M. has received research funding from Kyowa Kirin Co., Ltd.; consulting fees from Kyowa Kirin Co., Ltd. and Alexion Pharmaceuticals Inc.; and payment or honoraria from Kyowa Kirin Co., Ltd., Alexion Pharmaceuticals Inc., Amgen Inc., Chugai Pharmaceuticals Co., Ltd., and Novo Nordisk Pharma Ltd. H.G.K. has received grants or contracts from Chong Kun Dang pharmaceutical Corp., HANDOK Inc., Kyowa Kirin Co., Ltd., Amgen K.K., Apellis Pharmaceuticals, Inc., and AstraZeneca K.K.; consulting fees from HANDOK Inc. and Kyowa Kirin Co., Ltd.; payment or honoraria from Alexion Pharmaceuticals, Inc., HANDOK Inc., and Kyowa Kirin Co., Ltd.; and participated on a Data Safety Monitoring Board or Advisory Board of Bayer Yakuhin, Ltd. N.N. has received research funding, consulting fees, and payment or honoraria from Kyowa Kirin Co., Ltd. N.I. has received research funding and consulting fees from Kyowa Kirin Co., Ltd. T.K. has received research funding, consulting fees, and payment or honoraria from Kyowa Kirin Co., Ltd. A.S. has received research funding from Kyowa Kirin Co., Ltd.; consulting fees from Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Kyowa Kirin Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Shionogi & Co., Ltd.; and payment or honoraria from AbbVie G.K., Asahi Kasei Pharma Corporation, AstraZeneca K.K., Astellas Pharma Inc., Bayer Yakuhin, Ltd., Bristol-Myers Squibb K.K., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Janssen Pharmaceutical K.K., Kissei Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Mallinckrodt Pharma K.K., Merck Ltd., Maruho Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Nipro Corporation, Novo Nordisk Pharma Ltd., Nippon Shinyaku Co., Ltd., Ono Pharmaceutical Co., Ltd., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd., Taisho Pharmaceutical Co., Ltd., and Torii Pharmaceutical Co., Ltd. D.K. has received research funding and consulting fees from Kyowa Kirin Co., Ltd. S.F. has received research funding and consulting fees from Kyowa Kirin Co., Ltd. K.O. has received consulting fees, and payment or honoraria from Kyowa Kirin Co., Ltd. M.K. and Y.N. are employees of Kyowa Kirin Co., Ltd.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

