Benzylic C(sp3)-H fluorination
- PMID: 39015617
- PMCID: PMC11250007
- DOI: 10.3762/bjoc.20.137
Benzylic C(sp3)-H fluorination
Abstract
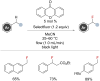
The selective fluorination of C(sp3)-H bonds is an attractive target, particularly for pharmaceutical and agrochemical applications. Consequently, over recent years much attention has been focused on C(sp3)-H fluorination, and several methods that are selective for benzylic C-H bonds have been reported. These protocols operate via several distinct mechanistic pathways and involve a variety of fluorine sources with distinct reactivity profiles. This review aims to give context to these transformations and strategies, highlighting the different tactics to achieve fluorination of benzylic C-H bonds.
Keywords: C–H functionalization; benzylic; fluorination; photoredox catalysis.
Copyright © 2024, Atkins et al.
Figures






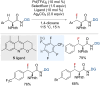


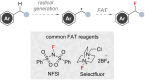



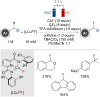









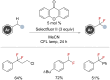


















References
Publication types
LinkOut - more resources
Full Text Sources
