Efficacy and Safety of High-Dose TBS on Poststroke Upper Extremity Motor Impairment: A Randomized Controlled Trial
- PMID: 39016009
- PMCID: PMC11346718
- DOI: 10.1161/STROKEAHA.124.046597
Efficacy and Safety of High-Dose TBS on Poststroke Upper Extremity Motor Impairment: A Randomized Controlled Trial
Abstract
Background: Upper extremity (UE) motor function impairment is a major poststroke complication whose recovery remains one of the most challenging tasks in neurological rehabilitation. This study examined the efficacy and safety of the personalized neuroimaging-guided high-dose theta-burst stimulation (TBS) for poststroke UE motor function recovery.
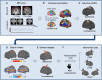
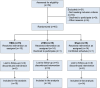
Methods: Patients after stroke with UE motor impairment from a China rehabilitation center were randomly assigned to receive high-dose intermittent TBS (iTBS) to ipsilesional UE sensorimotor network, continuous TBS (cTBS) to contralesional UE sensorimotor network, or sham stimulation, along with conventional therapy for 3 weeks. The primary outcome was the score changes on the Fugl-Meyer assessment-UE from baseline to 1 and 3 weeks. The secondary outcomes included the response rate on Fugl-Meyer assessment-UE scores posttreatment (≥9-point improvement) and score changes in multidimensional scales measuring UE, lower extremity, and activities and participation.
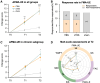
Results: From June 2021 to June 2022, 45 participants were randomized and 43 were analyzed. The iTBS and continuous TBS groups showed significantly greater improvement in Fugl-Meyer assessment-UE (mean improvement, iTBS: 10.73 points; continuous TBS: 10.79 points) than the sham group (2.43 points) and exhibited significantly greater response rates on Fugl-Meyer assessment-UE (iTBS, 60.0%; continuous TBS, 64.3%) than the sham group (0.0%). The active groups consistently exhibited superior improvement on the other 2 UE assessments at week 3. However, only the iTBS group showed greater efficacy on 1 lower extremity assessment than the sham group at week 3. Both active groups showed significant improvements in activities and participation assessments.
Conclusions: The study provides evidence for the efficacy and safety of high-dose TBS in facilitating poststroke UE rehabilitation.
Registration: URL: www.chictr.org.cn; Unique identifier: ChiCTR2100047340.
Keywords: magnetic resonance imaging; neurological rehabilitation; stroke; transcranial magnetic stimulation; upper extremity.
Conflict of interest statement
Dr Liu receives compensation from Neural Galaxy, Inc, for consultant services. R. Pan, M. Ding, and J. Sun are employees of the company, which is not a sponsor of this study. The other authors report no conflicts.
Figures
References
-
- Wyller TB, Sveen U, Sodring KM, Pettersen AM, Bautz-Holter E. Subjective well-being one year after stroke. Clin Rehabil. 1997;11:139–145. doi: 10.1177/026921559701100207 - PubMed
-
- Anwer S, Waris A, Gilani SO, Iqbal J, Shaikh N, Pujari AN, Niazi IK. Rehabilitation of upper limb motor impairment in stroke: a narrative review on the prevalence, risk factors, and economic statistics of stroke and state of the art therapies. Healthcare (Basel). 2022;10:190. doi: 10.3390/healthcare10020190 - PMC - PubMed
-
- Kwakkel G, Kollen BJ, van der Grond J, Prevo AJ. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34:2181–2186. doi: 10.1161/01.STR.0000087172.16305.CD - PubMed
-
- Wessel MJ, Egger P, Hummel FC. Predictive models for response to non-invasive brain stimulation in stroke: a critical review of opportunities and pitfalls. Brain Stimul. 2021;14:1456–1466. doi: 10.1016/j.brs.2021.09.006 - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

