Topical Cannabidiol for Established Chemotherapy-Induced Neuropathy: A Pilot Randomized Placebo-Controlled Trial
- PMID: 39016024
- PMCID: PMC11685298
- DOI: 10.1089/can.2023.0253
Topical Cannabidiol for Established Chemotherapy-Induced Neuropathy: A Pilot Randomized Placebo-Controlled Trial
Abstract
Background: Patients have been known to use cannabinoids for treating established chemotherapy-induced peripheral neuropathy (CIPN) based on anecdotal information and retrospective reports suggesting that such might be beneficial. In response, a double-blinded, placebo-controlled, randomized, pilot clinical trial was developed to evaluate whether resultant data would support a phase III trial for testing whether a cannabidiol (CBD) cream might improve CIPN. Methods: Forty patients with established CIPN were randomized, in a double-blinded manner, to topical CBD or a placebo cream. The study product was applied for 2 weeks, followed by a crossover for 2 weeks. Neuropathy was evaluated using the European Organization of Research and Treatment of Cancer (EORTC)-CIPN20, the Chemotherapy-Induced Peripheral Neuropathy Assessment Tool, and the Global Impression of Change instruments. Side effects were recorded by symptom diaries. Results: The EORTC-CIPN20 scores were similar in the patients receiving CBD versus the placebo. Likewise, the toxicity scores were similar in patients who received the CBD versus the placebo. Conclusions: This pilot trial did not support that the studied CBD isolate cream improved painful established CIPN. It was well tolerated overall. Clinical Trial Registration Number: NCT05388058.
Keywords: cannabidiol; chemotherapy-induced neuropathy; clinical trial; topical.
Figures
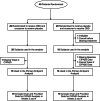
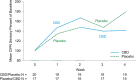


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

