Tocilizumab and CytoSorb for delayed severe cytokine release syndrome after ipilimumab plus nivolumab immunotherapy
- PMID: 39016056
- PMCID: PMC11457641
- DOI: 10.1080/1750743X.2024.2370180
Tocilizumab and CytoSorb for delayed severe cytokine release syndrome after ipilimumab plus nivolumab immunotherapy
Abstract
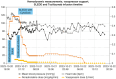
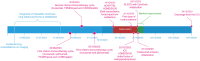
Cytokine release syndrome (CRS) is immune dysregulation phenomenon that is associated with immune checkpoint inhibitors. It is still difficult to distinguish CRS from other dangerous, acute and life-threatening medical disorders.We present a case of delayed grade 4 CRS following treatment of lung adenocarcinoma with ipilimumab plus nivolumab that warranted intensive care level treatment with abundant fluid resuscitation, two-tire vasopressor support, high-flow nasal oxygenation, corticosteroids in high dosages, as well as sustained low-efficiency daily diafiltration with CytoSorb hemadsorption and tocilizumab. Initial treatment of presumed septic shock of unknown origin did not yield results.After initiation of corticosteroids and particularly CytoSorb hemadsorption and tocilizumab, prompt clinical and laboratory improvement was observed.
Keywords: CytoSorb; critically ill; cytokine release syndrome; immune checkpoint inhibitors; intensive care unit; interleukin-6; tocilizumab.
Plain language summary
This case report describes a 62-year-old woman who experienced a life-threatening immune system reaction, 2 weeks after receiving immunotherapy for lung cancer. This reaction, called cytokine release syndrome (CRS), caused her organs to malfunction. The patient was treated with high-dose steroids, a blood purification technique (SLEDD with CytoSorb), and the medication tocilizumab. Her condition stabilized after initiation of SLEDD with CytoSorb and dramatically improved after receiving tocilizumab. This case highlights the importance of considering CRS in patients who experience severe illness after receiving immunotherapy.
Conflict of interest statement
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, stock ownership or options and expert testimony.
Figures

References
-
- Foran AE, Nadel HR, Lee AF, et al. Nivolumab in the treatment of refractory pediatric Hodgkin lymphoma. J Pediatr Hematol Oncol. 2017;39(5):e263–e266. doi: 10.1097/MPH.0000000000000703 - DOI - PubMed
-
• Represents the first published pediatric use of nivolumab for Hodgkin lymphoma, thus highlighting the use of checkpoint inhibitors (CPI) for hematological malignancies.
-
- Queirolo P, Boutros A, Tanda E, et al. Immune-checkpoint inhibitors for the treatment of metastatic melanoma: a model of cancer immunotherapy. Semin Cancer Biol. 2019;59:290–297. doi: 10.1016/j.semcancer.2019.08.001 - DOI - PubMed
-
• In this review, authors provided the review of introduction of CPIs in the treatment of malignant melanoma with data on improved survival (1-year survival with chemotherapy around 25%, with much improved survival in the case of immunotherapy or combinations – 3-year survival ranging between 12.2 and 58%).
-
- Hu Z, Li M, Chen Z, et al. Advances in clinical trials of targeted therapy and immunotherapy of lung cancer in 2018. Transl Lung Cancer Res. 2019;8(6):1091–1106. doi: 10.21037/tlcr.2019.10.17 - DOI - PMC - PubMed
-
• Highlights superior efficacy if immunotherapy compared to standard therapy for lung carcinomas (EGFR-TKIs are considered first-line treatment for EGFR positive carcinomas).
-
- Xu W, Atkins MB, McDermott DF. Checkpoint inhibitor immunotherapy in kidney cancer. Nat Rev Urol. 2020;17(3):137–150. doi: 10.1038/s41585-020-0282-3 - DOI - PubMed
-
• Similar review done for renal carcinomas examining different therapeutic approaches and providing US FDA approval status (e.g., nivolumab vs. everolimus for treatment of renal cell carcinoma, overall survival (OS) hazard ratio (HR) is 0.73).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
