Public health management of pertussis in adults: Practical challenges and future strategies
- PMID: 39016172
- PMCID: PMC11259069
- DOI: 10.1080/21645515.2024.2377904
Public health management of pertussis in adults: Practical challenges and future strategies
Abstract
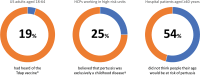
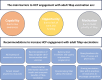
A panel of 24 international experts met in July 2022 to discuss challenges associated with pertussis detection, monitoring, and vaccination in adults; conclusions from this meeting are presented. There has been a shift in the epidemiology of pertussis toward older children and adults. This shift has been attributed to the waning of infection- or vaccine-induced immunity, newer detection techniques causing detection bias, and possibly the replacement of whole-cell pertussis with acellular vaccines in high-income countries, which may lead to immunity waning more quickly. The burden of adult pertussis is still likely under-ascertained due to widespread under-recognition by healthcare professionals (HCPs), under-diagnosis, and under-reporting in this age group. Non-standardized testing guidance and varied case definitions have contributed to under-reporting. Key barriers to HCP engagement with the tetanus, diphtheria, and pertussis (Tdap) vaccine include low awareness, lack of time/funding, and lack of motivation due to low prioritization of Tdap.
Keywords: Bordetella pertussis; Tdap vaccination; adults; comorbidities; disease burden; under-reporting.
Conflict of interest statement
C
Figures
References
-
- WHO . Pertussis: vaccine preventable diseases surveillance standards [Internet]. World Health Organization; 2018. [accessed 2023 Oct 6]. https://cdn.who.int/media/docs/default-source/immunization/vpd_surveilla....
-
- Fiona P, Havers M, Moro PL, Hariri S, Skoff T.. Pertussis ‘Pink Book’ [Internet]. 2021. [accessed 2023 Oct 6]. https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/pert.pdf.
-
- WHO . Pertussis reported cases by country [Internet]; 2022. [accessed 2023 Oct 6]. https://apps.who.int/gho/data/node.main.WHS3_43?lang=en.
-
- ECDC . Pertussis - Annual epidemiological report for 2018 [Internet]. European Centre for Disease Prevention and Control; 2020. [accessed 2023 Sep 7]. https://www.ecdc.europa.eu/en/publications-data/pertussis-annual-epidemi....
-
- CDC . 2021 final pertussis surveillance report [Internet]. Centres for Disease Control and Prevention; 2021. [accessed 2023 Aug 18]. https://www.cdc.gov/pertussis/downloads/pertuss-surv-report-2021.pdf.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous