Prevalence, Clinical Severity, and Serotype Distribution of Pneumococcal Pneumonia Among Adults Hospitalized With Community-Acquired Pneumonia in Tennessee and Georgia, 2018-2022
- PMID: 39016606
- PMCID: PMC11478805
- DOI: 10.1093/cid/ciae316
Prevalence, Clinical Severity, and Serotype Distribution of Pneumococcal Pneumonia Among Adults Hospitalized With Community-Acquired Pneumonia in Tennessee and Georgia, 2018-2022
Abstract
Introduction: Understanding the pneumococcal serotypes causing community-acquired pneumonia (CAP) is essential for evaluating the impact of pneumococcal vaccines.
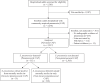
Methods: We conducted a prospective surveillance study of adults aged ≥18 years hospitalized with CAP at 3 hospitals in Tennessee and Georgia between 1 September 2018 and 31 October 2022. We assessed for pneumococcal etiology with cultures, the BinaxNOW urinary antigen detection test, and serotype-specific urinary antigen detection assays that detect 30 pneumococcal serotypes contained in the investigational pneumococcal conjugate vaccine V116, as well as licensed vaccines PCV15 and PCV20 (except serotype 15B). The distribution of pneumococcal serotypes was calculated based on serotype-specific urinary antigen detection results.
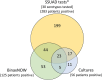
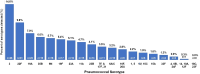
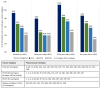
Results: Among 2917 hospitalized adults enrolled with CAP, 352 (12.1%) patients had Streptococcus pneumoniae detected, including 51 (1.7%) patients with invasive pneumococcal pneumonia. The 8 most commonly detected serotypes were: 3, 22F, 19A, 35B, 9N, 19F, 23A, and 11A. Among 2917 adults with CAP, 272 (9.3%) had a serotype detected that is contained in V116, compared to 196 (6.7%) patients with a serotype contained in PCV20 (P < .001), and 168 (5.8%) patients with a serotype contained in PCV15 (P < .001). A serotype contained in V116 but not PCV15 or PCV20 was detected in 120 (4.1%) patients, representing 38.0% of serotype detections.
Conclusions: Approximately 12% of adults hospitalized with CAP had S. pneumoniae detected, and approximately one-third of the detected pneumococcal serotypes were not contained in PCV15 or PCV20. Development of new pneumococcal vaccines with expanded serotype coverage has the potential to prevent a substantial burden of disease.
Keywords: Streptococcus pneumoniae; pneumococcus; pneumonia; serotype; vaccine.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest . This study was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc. Additionally, the following authors made the following disclosures. K. D. J. reports being an employee and shareholder with stock options of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc. C. G. G. reports consulting fees from Merck & Co., Inc and research funding outside the submitted work from NIH, CDC, AHRQ, FDA, and Campbell Alliance/Syneos Health. C. A. R. reports research funding outside the submitted work from BioFire, GSK, MedImmune, Micron, Merck, Novavx, PaxVax, Regeneron, Pfizer, Sanofi-Pasteur, Janssen, Moderna, NIH, and CDC, reports royalties from Meissa Vaccines, and reports 2 planned patents for RSV vaccines. I. Y. reports research funding outside the submitted work from Merck, and consulting fees from Sanofi Pasteur and Merck. T. W. reports being an employee and shareholder with stock options of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc. C. R. reports being an employee and shareholder with stock options of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc. N. R. reports research funding outside the submitted work from Merck, Sanofi, Pfizer, Vaccine Company, Immorna, Quidel and Lilly, reports consulting fees from Krog, reports honoraria from Virology Education and Medscape, reports support for attending a meeting from Sanofi and Moderna, reports participation in advisory boards for Moderna, Sanofi, Seqirus, Pfizer, EMMES, ICON, and Micron, and reports leadership roles with ARLG, TMRC, CDC-Pertussis challenge, and Clinical Infectious Diseases. The other authors reported no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Figures
References
-
- Ahmed SS, Pondo T, Xing W, et al. . Early impact of 13-valent pneumococcal conjugate vaccine use on invasive pneumococcal disease among adults with and without underlying medical conditions-United States. Clin Infect Dis 2020; 70:2484–92. - PubMed
-
- Kolditz M, Schmitt J, Pletz MW, Tesch F. Impact of the 13-valent pneumococcal conjugate vaccine on the incidence of all-cause pneumonia in adults aged ≥60 years: a population-based, retrospective cohort study. Clin Infect Dis 2019; 68:2117–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

