Chemical genetic screens reveal defective lysosomal trafficking as synthetic lethal with NF1 loss
- PMID: 39016685
- PMCID: PMC11361638
- DOI: 10.1242/jcs.262343
Chemical genetic screens reveal defective lysosomal trafficking as synthetic lethal with NF1 loss
Abstract
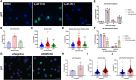
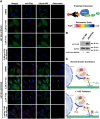
Neurofibromatosis type 1, a genetic disorder caused by pathogenic germline variations in NF1, predisposes individuals to the development of tumors, including cutaneous and plexiform neurofibromas (CNs and PNs), optic gliomas, astrocytomas, juvenile myelomonocytic leukemia, high-grade gliomas and malignant peripheral nerve sheath tumors (MPNSTs), which are chemotherapy- and radiation-resistant sarcomas with poor survival. Loss of NF1 also occurs in sporadic tumors, such as glioblastoma (GBM), melanoma, breast, ovarian and lung cancers. We performed a high-throughput screen for compounds that were synthetic lethal with NF1 loss, which identified several leads, including the small molecule Y102. Treatment of cells with Y102 perturbed autophagy, mitophagy and lysosome positioning in NF1-deficient cells. A dual proteomics approach identified BLOC-one-related complex (BORC), which is required for lysosome positioning and trafficking, as a potential target of Y102. Knockdown of a BORC subunit using siRNA recapitulated the phenotypes observed with Y102 treatment. Our findings demonstrate that BORC might be a promising therapeutic target for NF1-deficient tumors.
Keywords: BORC; Lysosomes; Mitochondria; NF1; RAS; Synthetic lethal.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures





References
-
- Al-Mehdi, A.-B., Pastukh, V. M., Swiger, B. M., Reed, D. J., Patel, M. R., Bardwell, G. C., Pastukh, V. V., Alexeyev, M. F. and Gillespie, M. N. (2012). Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci. Signal. 5, ra47. 10.1126/scisignal.2002712 - DOI - PMC - PubMed
-
- Alcantara Llaguno, S. R., Wang, Z., Sun, D., Chen, J., Xu, J., Kim, E., Hatanpaa, K. J., Raisanen, J. M., Burns, D. K., Johnson, J. E.et al. (2015). Adult lineage-restricted CNS progenitors specify distinct glioblastoma subtypes. Cancer Cell 28, 429-440. 10.1016/j.ccell.2015.09.007 - DOI - PMC - PubMed
-
- Allaway, R. J., Fischer, D. A., de Abreu, F. B., Gardner, T. B., Gordon, S. R., Barth, R. J., Colacchio, T. A., Wood, M., Kacsoh, B. Z., Bouley, S. J.et al. (2016). Genomic characterization of patient-derived xenograft models established from fine needle aspirate biopsies of a primary pancreatic ductal adenocarcinoma and from patient-matched metastatic sites. Oncotarget 7, 17087-17102. 10.18632/oncotarget.7718 - DOI - PMC - PubMed
-
- Allaway, R. J., Wood, M. D., Downey, S. L., Bouley, S. J., Traphagen, N. A., Wells, J. D., Batra, J., Melancon, S. N., Ringelberg, C., Seibel, W.et al. (2017). Exploiting mitochondrial and metabolic homeostasis as a vulnerability in NF1 deficient cells. Oncotarget 9, 15860-15875. 10.18632/oncotarget.19335 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Nancy P. Shea Trust
- Neurofibromatosis Therapeutic Acceleration Program
- S10 OD016212/OD/NIH HHS/United States
- T32 HG010464/HG/NHGRI NIH HHS/United States
- Congressionally Directed Medical Research Programs
- P30 CA023108/CA/NCI NIH HHS/United States
- W81XWH-16-1-0220/Department of Defense
- R01 NS095411/NS/NINDS NIH HHS/United States
- R21 NS060940/NS/NINDS NIH HHS/United States
- P20 GM113132/GM/NIGMS NIH HHS/United States
- U.S. National Science Foundation
- Friends of the Norris Cotton Cancer Center
- R35 GM145596/GM/NIGMS NIH HHS/United States
- R01 GM122846/GM/NIGMS NIH HHS/United States
- Children's Tumor Foundation
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

