Targeting AKR1B10 by Drug Repurposing with Epalrestat Overcomes Chemoresistance in Non-Small Cell Lung Cancer Patient-Derived Tumor Organoids
- PMID: 39017606
- PMCID: PMC11369614
- DOI: 10.1158/1078-0432.CCR-23-3980
Targeting AKR1B10 by Drug Repurposing with Epalrestat Overcomes Chemoresistance in Non-Small Cell Lung Cancer Patient-Derived Tumor Organoids
Abstract
Purpose: Systemic treatments given to patients with non-small cell lung cancer (NSCLC) are often ineffective due to drug resistance. In the present study, we investigated patient-derived tumor organoids (PDTO) and matched tumor tissues from surgically treated patients with NSCLC to identify drug repurposing targets to overcome resistance toward standard-of-care platinum-based doublet chemotherapy.
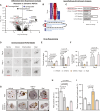
Experimental design: PDTOs were established from 10 prospectively enrolled patients with non-metastatic NSCLC from resected tumors. PDTOs were compared with matched tumor tissues by histopathology/immunohistochemistry, whole exome sequencing, and transcriptome sequencing. PDTO growths and drug responses were determined by measuring 3D tumoroid volumes, cell viability, and proliferation/apoptosis. Differential gene expression analysis identified drug-repurposing targets. Validations were performed with internal/external data sets of patients with NSCLC. NSCLC cell lines were used for aldo-keto reductase 1B10 (AKR1B10) knockdown studies and xenograft models to determine the intratumoral bioavailability of epalrestat.
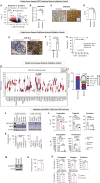
Results: PDTOs retained histomorphology and pathological biomarker expression, mutational/transcriptomic signatures, and cellular heterogeneity of the matched tumor tissues. Five (50%) PDTOs were chemoresistant toward carboplatin/paclitaxel. Chemoresistant PDTOs and matched tumor tissues demonstrated overexpression of AKR1B10. Epalrestat, an orally available AKR1B10 inhibitor in clinical use for diabetic polyneuropathy, was repurposed to overcome chemoresistance of PDTOs. In vivo efficacy of epalrestat to overcome drug resistance corresponded to intratumoral epalrestat levels.
Conclusions: PDTOs are efficient preclinical models recapitulating the tumor characteristics and are suitable for drug testing. AKR1B10 can be targeted by repurposing epalrestat to overcome chemoresistance in NSCLC. Epalrestat has the potential to advance to clinical trials in patients with drug-resistant NSCLC due to favorable toxicity, pharmacological profile, and bioavailability.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
M.A. Ciorba reports grants from Incyte, Pfizer, and Janssen as well as personal fees from AbbVie and from Geneoscopy outside the submitted work. J.T. Kaifi reports grants from Department of Veterans Affairs during the conduct of the study as well as other support from Extract Biologics, LLC, outside the submitted work; in addition, J.T. Kaifi has a patent for US 11,890,616B2 issued and licensed to Kaifi/Kwon. No disclosures were reported by the other authors.
Figures


References
-
- Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon J-P, Vansteenkiste J, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351–60. - PubMed
-
- Krebs MG, Sloane R, Priest L, Lancashire L, Hou J-M, Greystoke A, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 2011;29:1556–63. - PubMed
-
- Wu Y-L, Tsuboi M, He J, John T, Grohe C, Majem M, et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med 2020;383:1711–23. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

