Numb-associated kinases regulate sandfly-borne Toscana virus entry
- PMID: 39017647
- PMCID: PMC11285224
- DOI: 10.1080/22221751.2024.2382237
Numb-associated kinases regulate sandfly-borne Toscana virus entry
Abstract
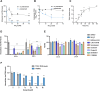
Sandfly-borne Toscana virus (TOSV) is an enveloped tri-segmented negative single-strand RNA Phlebovirus. It is an emerging virus predominantly endemic in southwestern Europe and Northern Africa. Although TOSV infection is typically asymptomatic or results in mild febrile disease, it is neurovirulent and ranks among the three most common causes of summer meningitis in certain regions. Despite this clinical significance, our understanding of the molecular aspects and host factors regulating phlebovirus infection is limited. This study characterized the early steps of TOSV infection. Our findings reveal that two members of the Numb-associated kinases family of Ser/Thr kinases, namely adaptor-associated kinase 1 (AAK1) and cyclin G-associated kinase (GAK), play a role in regulating the early stages of TOSV entry. FDA-approved inhibitors targeting these kinases demonstrated significant inhibition of TOSV infection. This study suggests that AAK1 and GAK represent druggable targets for inhibiting TOSV infection and, potentially, related Phleboviruses.
Keywords: Bunyaviruses; Phleboviruses; Sandfly; Toscana virus; viral entry.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
-
- Briese T, Calisher CH, Higgs S.. Viruses of the family Bunyaviridae: are all available isolates reassortants? Virology. 2013;446(1–2):207–216. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
