Novel Combinations of Immunotherapies or DNA Damage Repair Inhibitors in Platinum-Refractory Extensive-Stage Small Cell Lung Cancer: The Phase II BALTIC Study
- PMID: 39017667
- PMCID: PMC11393542
- DOI: 10.1158/1078-0432.CCR-24-0013
Novel Combinations of Immunotherapies or DNA Damage Repair Inhibitors in Platinum-Refractory Extensive-Stage Small Cell Lung Cancer: The Phase II BALTIC Study
Abstract
Purpose: The phase II, multiarm, signal-searching BALTIC study (NCT02937818) assessed novel treatment combinations for platinum-refractory/resistant extensive-stage small cell lung cancer (ES-SCLC).
Patients and methods: Patients with ES-SCLC with progressive disease during or within 90 days of completing first-line platinum-based chemotherapy received one of three regimens: durvalumab plus tremelimumab followed by durvalumab monotherapy (arm A), adavosertib plus carboplatin (arm B), or ceralasertib plus olaparib (arm C). The primary endpoint was the objective response rate. Prespecified exploratory biomarker analyses were conducted in arms A and C.
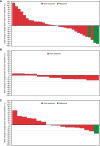
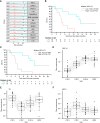
Results: In arm A (n = 41), arm B (n = 10), and arm C (n = 21), the confirmed objective response rates were 7.3%, 0%, and 4.8%, respectively. Safety profiles in all arms were consistent with those of the individual drugs. In arm A, patients with PD-L1 expression (tumor cells or immune cells) ≥1% seemed to have a greater likelihood of achieving disease control with durvalumab plus tremelimumab than those with PD-L1 (tumor cells and immune cells) <1%, and lower baseline ctDNA and reduction in the on-treatment ctDNA level were both associated with longer overall survival. Among patients treated with ceralasertib plus olaparib in arm C, specific immune response-relevant circulating chemokines and cytokines were identified as early biomarkers of survival and pharmacodynamic biomarkers.
Conclusions: In BALTIC, all combination regimens demonstrated tolerable safety profiles, but antitumor activity was limited in refractory/resistant ES-SCLC. Among patients treated with durvalumab plus tremelimumab, an association of on-treatment reduction in ctDNA with longer overall survival suggests the potential use of ctDNA as a surrogate of treatment response, warranting further investigation.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
N. Reinmuth reports personal fees from Amgen, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, GSK, Hoffmann-La Roche, Janssen, MSD, Pfizer, Asklepios GmbH Gauting, Boehringer Ingelheim, Eli Lilly and Company, Sanofi, and Takeda outside the submitted work. O.J. Juan-Vidal reports personal fees from AstraZeneca, Bristol Myers Squibb, Johnson & Johnson, Eli Lilly and Company, Merck Sharp & Dohme, Roche/Genentech, and Takeda outside the submitted work. M. Bryl reports personal fees from AstraZeneca during the conduct of the study, as well as personal fees from AstraZeneca, Roche/Genentech, MSD, Bristol Myers Squibb, Boehringer Ingelheim, Takeda, Novartis, Sanofi, and Pfizer outside the submitted work. D. Vicente reports personal fees from AstraZeneca, Roche, Pfizer, Novartis, Bristol Myers Squibb, Takeda, Eli Lilly and Company, and MSD outside the submitted work. J. Armstrong reports personal fees and other support from AstraZeneca during the conduct of the study, as well as personal fees and other support from AstraZeneca outside the submitted work. T. Dalvi reports other support from AstraZeneca during the conduct of the study; other support from AstraZeneca outside the submitted work; and employment with AstraZeneca and ownership of stock. M. Xie reports personal fees from AstraZeneca during the conduct of the study. S. Iyer reports other support from AstraZeneca during the conduct of the study, as well as other support from AstraZeneca outside the submitted work. Y. Shrestha reports other support from AstraZeneca outside the submitted work. H. Jiang reports employment with AstraZeneca, which is the sponsor of the trial. No disclosures were reported by the other authors.
Figures

References
-
- Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019;394:1929–39. - PubMed
-
- Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018;379:2220–9. - PubMed
-
- von Pawel J, Jotte R, Spigel DR, O’Brien MER, Socinski MA, Mezger J, et al. Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J Clin Oncol 2014;32:4012–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

