Phase 3 THOR Japanese subgroup analysis: erdafitinib in advanced or metastatic urothelial cancer and fibroblast growth factor receptor alterations
- PMID: 39017806
- PMCID: PMC11420312
- DOI: 10.1007/s10147-024-02583-3
Phase 3 THOR Japanese subgroup analysis: erdafitinib in advanced or metastatic urothelial cancer and fibroblast growth factor receptor alterations
Abstract
Background: In the THOR trial (NCT03390504) Cohort 1, erdafitinib demonstrated significantly prolonged overall survival (OS) (median 12.1 versus 7.8 months) and reduced risk of death by 36% (hazard ratio 0.64, P = 0.005) compared with chemotherapy in metastatic urothelial carcinoma (mUC) patients with FGFR alterations who progressed after ≥ 1 prior treatments, including anti-PD-(L)1. There have been no reports of the Japanese subgroup results yet.
Methods: THOR Cohort 1 randomized patients to erdafitinib once daily or docetaxel/vinflunine once every 3 weeks. Primary endpoint was OS. Secondary endpoints included progression-free survival (PFS) and objective response rate (ORR). No specific statistical power was set for this Japanese subgroup analysis.
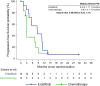
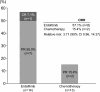
Results: Of 266 patients randomized, 27 (14 erdafitinib; 13 chemotherapy) were Japanese. Baseline characteristics were generally similar between treatments and to the overall population, except for more males, lower body weight, and more upper tract primary tumors among Japanese patients. Compared with chemotherapy, erdafitinib showed improved OS (median 25.4 versus 12.4 months), PFS (median 8.4 versus 2.9 months) and ORR (57.1% versus 15.4%). Any grade treatment-related adverse events (AEs) occurred in all patients from both arms but Grade 3/4 AEs and AEs leading to discontinuation were lower in the erdafitinib arm. No new safety signals were observed in the Japanese subgroup.
Conclusion: In the Japanese subgroup, erdafitinib showed improved survival and response compared to chemotherapy, with no new safety concerns. These results support erdafitinib as a treatment option for Japanese mUC patients with FGFR alterations, and early FGFR testing after diagnosis of mUC should be considered.
Keywords: Erdafitinib; Fibroblast growth factor receptor; Japanese subgroup; Metastatic urothelial carcinoma; Oral tyrosine kinase inhibitor.
© 2024. The Author(s).
Conflict of interest statement
Nobuaki Matsubara has received grants or contracts from Janssen Pharmaceutical K.K., MSD K.K., Bayer Yakuhin, Ltd., Chugai Pharmaceutical Co., Ltd., AstraZeneca K.K., Astellas Pharma Inc., Bayer AG, Amgen K.K., Takeda Pharmaceutical Co. Ltd., Eli Lilly Japan K.K., Eisai Co. Ltd., Roche/Genentech, Seagen, Novartis Pharma K.K., and AbbVie Inc.; consulting fees from Sanofi K.K., Janssen Pharmaceutical K.K., AstraZeneca K.K., Eli Lilly Japan K.K., Amgen K.K., Seagen, and Pfizer Japan Inc.; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Sanofi K.K.. Yuji Miura has received grants or contracts from MSD and Ono Pharmaceutical Co., Ltd.; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Takeda Pharmaceutical Co., Ltd., Bristol-Myers Squibb K.K., Eisai Co. Ltd., Ono Pharmaceutical Co., Ltd., and MSD. Hiroyuki Nishiyama has received grants or contracts from Ono Pharmaceutical Co., Ltd., and Chugai Pharmaceutical Co., Ltd.; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from MSD, Astellas Pharma Inc., Merck Biopharma Co. Ltd., AstraZeneca K.K., Ono Pharmaceutical Co., Ltd., Bristol-Myers Squibb K.K., and Nippon Fine Chemical Co., Ltd..; other financial or non-financial interests from Bayer Yakuhin, Ltd Rikiya Taoka and Nobuaki Shimizu report no conflicts of interest. Takahiro Kojima has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Astellas Pharma Inc. Jason Hwang reports employment and employee stock ownership from Janssen Pharmaceutical K.K.; Tatsuya Ote reports employment from Janssen Pharmaceutical K.K., and employee stock ownership from Janssen Pharmaceutical K.K., and Johnson & Johnson. Ryo Oyama reports employment and employee stock ownership from Janssen Pharmaceutical K.K. Kiichiro Toyoizumi reports employment from Janssen Pharmaceutical K.K., and employee stock ownership from Johnson & Johnson. Sutapa Mukhopadhyay, Spyros Triantos, and Kris Deprince report employment from Janssen Research & Development, and employee stock ownership from Johnson & Johnson. Yohann Loriot has received grants or contracts from Janssen Oncology, MSD Oncology, AstraZeneca, Exelixis, Incyte, Pfizer Japan Inc., Sanofi K.K., Seagen, Astellas Pharma Inc., Gilead Sciences Inc., Merck KGaA, Taiho Pharmaceutical Co., Ltd., Bristol-Myers Squibb, Roche, and Tyra Biosciences; consulting fees from Janssen Pharmaceutical Co. Ltd., Astellas Pharma Inc., Roche, AstraZeneca, MSD Oncology, Seagen, Bristol-Myers Squibb, Taiho Pharmaceutical Co., Ltd., Loxo/Lilly, Pfizer/EMD Serono, Merck KGaA, Gilead Sciences, Pfizer Japan Inc., Roche, MSD Oncology; support for attending meetings and/or travel from Astellas Pharma, Janssen Oncology, Roche, MSD Oncology, AstraZeneca, and Seagen.
Figures
References
-
- Kaseb H, Aeddula N Bladder Cancer. [Updated 2022 Oct 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK536923/. Accessed 19 Oct 2023
-
- Cumberbatch MGK, Jubber I, Black PC et al (2018) Epidemiology of bladder cancer: A systematic review and contemporary update of risk factors in 2018. Eur Urol 74(6):784–795. 10.1016/j.eururo.2018.09.001 - PubMed
-
- Bharmal M, Guenther S, Kearney M (2017) Epidemiology of locally advanced or metastatic urothelial cancer in the US Europe and Japan. Value Health 20(9):A419. 10.1016/j.jval.2017.08.127
-
- National cancer center in-hospital cancer registries nationwide tally [In Japanese]. Available at: https://ganjoho.jp/public/qa_links/report/hosp_c/hosp_c_registry.html. Accessed 21 Nov 2023
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources