Chimeric Cell Therapy Transfers Healthy Donor Mitochondria in Duchenne Muscular Dystrophy
- PMID: 39017908
- PMCID: PMC11445288
- DOI: 10.1007/s12015-024-10756-w
Chimeric Cell Therapy Transfers Healthy Donor Mitochondria in Duchenne Muscular Dystrophy
Abstract
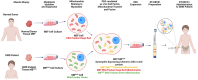
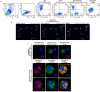
Duchenne muscular dystrophy (DMD) is a severe X-linked disorder characterized by dystrophin gene mutations and mitochondrial dysfunction, leading to progressive muscle weakness and premature death of DMD patients. We developed human Dystrophin Expressing Chimeric (DEC) cells, created by the fusion of myoblasts from normal donors and DMD patients, as a foundation for DT-DEC01 therapy for DMD. Our preclinical studies on mdx mouse models of DMD revealed enhanced dystrophin expression and functional improvements in cardiac, respiratory, and skeletal muscles after systemic intraosseous DEC administration. The current study explored the feasibility of mitochondrial transfer and fusion within the created DEC cells, which is crucial for developing new therapeutic strategies for DMD. Following mitochondrial staining with MitoTracker Deep Red and MitoTracker Green dyes, mitochondrial fusion and transfer was assessed by Flow cytometry (FACS) and confocal microscopy. The PEG-mediated fusion of myoblasts from normal healthy donors (MBN/MBN) and normal and DMD-affected donors (MBN/MBDMD), confirmed the feasibility of myoblast and mitochondrial fusion and transfer. The colocalization of the mitochondrial dyes MitoTracker Deep Red and MitoTracker Green confirmed the mitochondrial chimeric state and the creation of chimeric mitochondria, as well as the transfer of healthy donor mitochondria within the created DEC cells. These findings are unique and significant, introducing the potential of DT-DEC01 therapy to restore mitochondrial function in DMD patients and in other diseases where mitochondrial dysfunction plays a critical role.
Keywords: Chimeric mitochondria; DMD therapy; Dystrophin expressing chimeric (DEC) cells; Mitochondria in DMD; Mitochondrial fusion; Mitochondrial transfer.
© 2024. The Author(s).
Conflict of interest statement
MS is CMO and shareholder of Dystrogen Therapeutics Technology Polska z o.o. the company that holds a license for DT-DEC01 therapy. MS is the inventor on the patent application filed by University of Illinois at Chicago related to chimeric cell therapy for Duchenne muscular dystrophy (WO/2016/201182). The author declares a potential conflict of interest. KS is CEO and shareholder of Dystrogen Therapeutics Corp. The author declares a potential conflict of interest. KB, AZ, KJ are Dystrogen Therapeutics Corp. employees.
Figures

Similar articles
-
Transplantation of Dystrophin Expressing Chimeric Human Cells of Myoblast/Mesenchymal Stem Cell Origin Improves Function in Duchenne Muscular Dystrophy Model.Stem Cells Dev. 2021 Feb;30(4):190-202. doi: 10.1089/scd.2020.0161. Epub 2021 Jan 22. Stem Cells Dev. 2021. PMID: 33349121
-
Long-Term Protective Effect of Human Dystrophin Expressing Chimeric (DEC) Cell Therapy on Amelioration of Function of Cardiac, Respiratory and Skeletal Muscles in Duchenne Muscular Dystrophy.Stem Cell Rev Rep. 2022 Dec;18(8):2872-2892. doi: 10.1007/s12015-022-10384-2. Epub 2022 May 19. Stem Cell Rev Rep. 2022. PMID: 35590083 Free PMC article.
-
Human dystrophin expressing chimeric (DEC) cell therapy ameliorates cardiac, respiratory, and skeletal muscle's function in Duchenne muscular dystrophy.Stem Cells Transl Med. 2021 Oct;10(10):1406-1418. doi: 10.1002/sctm.21-0054. Epub 2021 Jul 22. Stem Cells Transl Med. 2021. PMID: 34291884 Free PMC article.
-
Chimeric Cell Therapies as a Novel Approach for Duchenne Muscular Dystrophy (DMD) and Muscle Regeneration.Biomolecules. 2024 May 13;14(5):575. doi: 10.3390/biom14050575. Biomolecules. 2024. PMID: 38785982 Free PMC article. Review.
-
Molecular correction of Duchenne muscular dystrophy by splice modulation and gene editing.RNA Biol. 2021 Jul;18(7):1048-1062. doi: 10.1080/15476286.2021.1874161. Epub 2021 Jan 20. RNA Biol. 2021. PMID: 33472516 Free PMC article. Review.
Cited by
-
Mechanisms of Chimeric Cell Therapy in Duchenne Muscular Dystrophy.Biomedicines. 2024 Sep 2;12(9):1996. doi: 10.3390/biomedicines12091996. Biomedicines. 2024. PMID: 39335509 Free PMC article.
-
Induced Pluripotent (iPSC) and Mesenchymal (MSC) Stem Cells for In Vitro Disease Modeling and Regenerative Medicine.Int J Mol Sci. 2025 Jun 11;26(12):5617. doi: 10.3390/ijms26125617. Int J Mol Sci. 2025. PMID: 40565081 Free PMC article. Review.
References
-
- Skuk, D., Goulet, M., Roy, B., Piette, V., Côté, C. H., Chapdelaine, P., Hogrel, J. Y., Paradis, M., Bouchard, J. P., Sylvain, M., et al. (2007). First Test of a high-density injection protocol for myogenic cell transplantation throughout large volumes of muscles in a Duchenne muscular dystrophy patient: Eighteen months Follow-Up. Neuromuscular Disorders, 17, 38–46. 10.1016/j.nmd.2006.10.003 - PubMed
-
- Hughes, S. M., & Blau, H. M. (1990). Migration of myoblasts across Basal Lamina during skeletal muscle development. Nature, 345, 350–353. 10.1038/345350a0 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

