Lipoprotein(a) and Long-Term Plaque Progression, Low-Density Plaque, and Pericoronary Inflammation
- PMID: 39018040
- PMCID: PMC11255968
- DOI: 10.1001/jamacardio.2024.1874
Lipoprotein(a) and Long-Term Plaque Progression, Low-Density Plaque, and Pericoronary Inflammation
Erratum in
-
Error in Abstract.JAMA Cardiol. 2024 Sep 1;9(9):861. doi: 10.1001/jamacardio.2024.3130. JAMA Cardiol. 2024. PMID: 39259261 Free PMC article. No abstract available.
Abstract
Importance: Lipoprotein(a) (Lp[a]) is a causal risk factor for cardiovascular disease; however, long-term effects on coronary atherosclerotic plaque phenotype, high-risk plaque formation, and pericoronary adipose tissue inflammation remain unknown.
Objective: To investigate the association of Lp(a) levels with long-term coronary artery plaque progression, high-risk plaque, and pericoronary adipose tissue inflammation.
Design, setting, and participants: This single-center prospective cohort study included 299 patients with suspected coronary artery disease (CAD) who underwent per-protocol repeated coronary computed tomography angiography (CCTA) imaging with an interscan interval of 10 years. Thirty-two patients were excluded because of coronary artery bypass grafting, resulting in a study population of 267 patients. Data for this study were collected from October 2008 to October 2022 and analyzed from March 2023 to March 2024.
Exposures: The median scan interval was 10.2 years. Lp(a) was measured at follow-up using an isoform-insensitive assay. CCTA scans were analyzed with a previously validated artificial intelligence-based algorithm (atherosclerosis imaging-quantitative computed tomography).
Main outcome and measures: The association between Lp(a) and change in percent plaque volumes was investigated in linear mixed-effects models adjusted for clinical risk factors. Secondary outcomes were presence of low-density plaque and presence of increased pericoronary adipose tissue attenuation at baseline and follow-up CCTA imaging.
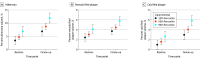
Results: The 267 included patients had a mean age of 57.1 (SD, 7.3) years and 153 were male (57%). Patients with Lp(a) levels of 125 nmol/L or higher had twice as high percent atheroma volume (6.9% vs 3.0%; P = .01) compared with patients with Lp(a) levels less than 125 nmol/L. Adjusted for other risk factors, every doubling of Lp(a) resulted in an additional 0.32% (95% CI, 0.04-0.60) increment in percent atheroma volume during the 10 years of follow-up. Every doubling of Lp(a) resulted in an odds ratio of 1.23 (95% CI, 1.00-1.51) and 1.21 (95% CI, 1.01-1.45) for the presence of low-density plaque at baseline and follow-up, respectively. Patients with higher Lp(a) levels had increased pericoronary adipose tissue attenuation around both the right coronary artery and left anterior descending at baseline and follow-up.
Conclusions and relevance: In this long-term prospective serial CCTA imaging study, higher Lp(a) levels were associated with increased progression of coronary plaque burden and increased presence of low-density noncalcified plaque and pericoronary adipose tissue inflammation. These data suggest an impact of elevated Lp(a) levels on coronary atherogenesis of high-risk, inflammatory, rupture-prone plaques over the long term.
Conflict of interest statement
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

