Systemic Immunomodulatory Treatments for Atopic Dermatitis: Living Systematic Review and Network Meta-Analysis Update
- PMID: 39018058
- PMCID: PMC11255974
- DOI: 10.1001/jamadermatol.2024.2192
Systemic Immunomodulatory Treatments for Atopic Dermatitis: Living Systematic Review and Network Meta-Analysis Update
Erratum in
-
Error in Studies Included.JAMA Dermatol. 2024 Sep 1;160(9):1012. doi: 10.1001/jamadermatol.2024.3600. JAMA Dermatol. 2024. PMID: 39292474 Free PMC article. No abstract available.
Abstract
Importance: There are multiple approved systemic treatments for atopic dermatitis. Lebrikizumab is a newly licensed biologic medication that has been compared to placebo in clinical trials but not to other systemic treatments.
Objective: To compare reported measures of efficacy and safety of lebrikizumab to other systemic treatments for atopic dermatitis in a living systematic review and network meta-analysis.
Data sources: The Cochrane Central Register of Controlled Trials, MEDLINE, Embase, the Latin American and Caribbean Health Science Information database, the Global Resource of Eczema Trials database, and trial registries were searched from inception through November 3, 2023.
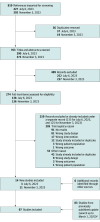
Study selection: Randomized clinical trials evaluating 8 or more weeks of treatment with systemic immunomodulatory medications for moderate to severe atopic dermatitis. Titles, abstracts, and full texts were screened in duplicate.
Data extraction and synthesis: Data were abstracted in duplicate and random-effects bayesian network meta-analyses were performed. Minimal important differences were used to define important differences between medications. Certainty of evidence was assessed using the GRADE approach (Grading of Recommendations Assessment, Development and Evaluation). The updated analysis was completed from December 13, 2023, to February 20, 2024.
Main outcome measures: Efficacy outcomes were the Eczema Area and Severity Index (EASI), the Patient Oriented Eczema Measure (POEM) Dermatology Life Quality Index (DLQI), and Peak Pruritus Numeric Rating Scales (PP-NRS) and were compared using mean difference (MD) with 95% credible intervals (CrI). Safety outcomes were serious adverse events and withdrawal due to adverse events. Other outcomes included the proportion of participants with 50%, 75%, and 90% improvement in EASI (EASI-50, -75, -90) and the proportion with success on the Investigator Global Assessment compared using odds ratios with 95% CrI.
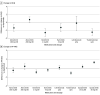
Results: The study sample included 97 eligible trials, with a total of 24 679 patients. Lebrikizumab was associated with no important difference in change in EASI (MD, -2.0; 95% CrI, -4.5 to 0.3; moderate certainty), POEM (MD, -1.1; 95% CrI -2.5 to 0.2; moderate certainty), DLQI (MD, -0.2; 95% CrI -2.1 to 1.6; moderate certainty), or PP-NRS (MD, 0.1; 95% CrI -0.4, 0.6; high certainty) compared to dupilumab among adults with atopic dermatitis who were treated for up to 16 weeks. Dupilumab was associated with higher odds of efficacy in binary outcomes compared with lebrikizumab. The relative efficacy of other approved systemic medications was similar to that found by previous updates of this living study, with high-dose upadacitinib and abrocitinib demonstrating numerically highest relative efficacy. For safety outcomes, low event rates limited useful comparisons.
Conclusions and relevance: In this living systematic review and network meta-analysis, lebrikizumab was similarly effective to dupilumab for the short-term treatment of atopic dermatitis in adults. Clinicians and patients can use these comparative data to inform treatment decisions.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

