Multinational Drug Survival Study of Omalizumab in Patients With Chronic Urticaria and Potential Predictors for Discontinuation
- PMID: 39018068
- PMCID: PMC11255966
- DOI: 10.1001/jamadermatol.2024.2056
Multinational Drug Survival Study of Omalizumab in Patients With Chronic Urticaria and Potential Predictors for Discontinuation
Erratum in
-
Error in Figure.JAMA Dermatol. 2024 Nov 1;160(11):1257. doi: 10.1001/jamadermatol.2024.4573. JAMA Dermatol. 2024. PMID: 39412760 Free PMC article. No abstract available.
Abstract
Importance: Treating patients with chronic urticaria using omalizumab has been shown to be safe and effective in randomized clinical trials. Multinational studies on long-term omalizumab performance in chronic urticaria in clinical practice settings are lacking, especially on drug survival. Drug survival, which refers to the length of time that patients are treated with a specific drug, is a comprehensive outcome covering effectiveness, safety, and patient and physician preferences. Furthermore, little is known about the reasons and potential predictors for omalizumab discontinuation.
Objective: To investigate omalizumab drug survival as well as reasons and potential predictors for discontinuation in a large, diverse population.
Design, setting, and participants: This international multicenter cohort study was conducted at 14 Urticaria Centers of Reference and Excellence in 10 countries, including all patients with chronic urticaria from these centers who were ever treated with omalizumab.
Main outcomes and measures: Drug survival analysis was performed to assess time to discontinuation. Patient characteristics and treatment protocols were investigated by Cox regression analysis to identify potential predictors for omalizumab discontinuation.
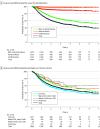
Results: In 2325 patients with chronic urticaria who started omalizumab between June 2009 and July 2022, the mean (SD) age of the cohort was 42 (6) years, and 1650 participants (71%) were female. Overall omalizumab survival rates decreased from 76% to 39% after 1 to 7 years, respectively (median survival time, 3.3 [95 % CI, 2.9-4.0] years), primarily due to discontinuation from well-controlled disease in 576 patients (65%). Ineffectiveness and adverse effects were reasons for discontinuation in a far smaller proportion of patients, totaling 164 patients (18%) and 31 patients (4%), respectively. Fast treatment response was associated with higher rates of omalizumab discontinuation due to well-controlled disease (hazard ratio, 1.45 [95% CI, 1.20-1.75]), and disease duration of more than 2 years was associated with lower rates of discontinuation due to well-controlled disease (HR, 0.81 [95% CI, 0.67-0.98]). Immunosuppressive cotreatment at the start of omalizumab and autoimmune disease was associated with a higher risk for discontinuation due to ineffectiveness (HR, 1.65 [95% CI, 1.12-2.42]). The presence of spontaneous wheals (HR, 0.62 [95% CI, 0.41-0.93]) and access to higher dosages (HR, 0.40 [95% CI, 0.27-0.58) were both associated with a lower risk for discontinuation of omalizumab due to ineffectiveness.
Conclusion and relevance: This multinational omalizumab drug survival cohort study demonstrated that treatment of chronic urticaria with omalizumab in a clinical setting is effective and safe, and well-controlled disease is the main reason for treatment discontinuation. These findings on omalizumab drug survival rates and reasons and potential predictors for discontinuation may guide patients and physicians in clinical decision-making and expectation management. These results may call for the identification of biomarkers for chronic urticaria remission in complete responders to omalizumab treatment.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

