Endogenous retroviruses mediate transcriptional rewiring in response to oncogenic signaling in colorectal cancer
- PMID: 39018396
- PMCID: PMC466953
- DOI: 10.1126/sciadv.ado1218
Endogenous retroviruses mediate transcriptional rewiring in response to oncogenic signaling in colorectal cancer
Abstract
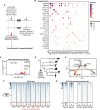
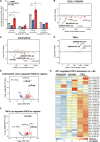
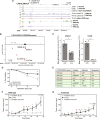
Cancer cells exhibit rewired transcriptional regulatory networks that promote tumor growth and survival. However, the mechanisms underlying the formation of these pathological networks remain poorly understood. Through a pan-cancer epigenomic analysis, we found that primate-specific endogenous retroviruses (ERVs) are a rich source of enhancers displaying cancer-specific activity. In colorectal cancer and other epithelial tumors, oncogenic MAPK/AP1 signaling drives the activation of enhancers derived from the primate-specific ERV family LTR10. Functional studies in colorectal cancer cells revealed that LTR10 elements regulate tumor-specific expression of multiple genes associated with tumorigenesis, such as ATG12 and XRCC4. Within the human population, individual LTR10 elements exhibit germline and somatic structural variation resulting from a highly mutable internal tandem repeat region, which affects AP1 binding activity. Our findings reveal that ERV-derived enhancers contribute to transcriptional dysregulation in response to oncogenic signaling and shape the evolution of cancer-specific regulatory networks.
Figures



References
-
- Rheinbay E., Nielsen M. M., Abascal F., Wala J. A., Shapira O., Tiao G., Hornshøj H., Hess J. M., Juul R. I., Lin Z., Feuerbach L., Sabarinathan R., Madsen T., Kim J., Mularoni L., Shuai S., Lanzós A., Herrmann C., Maruvka Y. E., Shen C., Amin S. B., Bandopadhayay P., Bertl J., Boroevich K. A., Busanovich J., Carlevaro-Fita J., Chakravarty D., Chan C. W. Y., Craft D., Dhingra P., Diamanti K., Fonseca N. A., Gonzalez-Perez A., Guo Q., Hamilton M. P., Haradhvala N. J., Hong C., Isaev K., Johnson T. A., Juul M., Kahles A., Kahraman A., Kim Y., Komorowski J., Kumar K., Kumar S., Lee D., Lehmann K.-V., Li Y., Liu E. M., Lochovsky L., Park K., Pich O., Roberts N. D., Saksena G., Schumacher S. E., Sidiropoulos N., Sieverling L., Sinnott-Armstrong N., Stewart C., Tamborero D., Tubio J. M. C., Umer H. M., Uusküla-Reimand L., Wadelius C., Wadi L., Yao X., Zhang C.-Z., Zhang J., Haber J. E., Hobolth A., Imielinski M., Kellis M., Lawrence M. S., von Mering C., Nakagawa H., Raphael B. J., Rubin M. A., Sander C., Stein L. D., Stuart J. M., Tsunoda T., Wheeler D. A., Johnson R., Reimand J., Gerstein M., Khurana E., Campbell P. J., López-Bigas N.; PCAWG Drivers and Functional Interpretation Working Group; PCAWG Structural Variation Working Group, Weischenfeldt J., Beroukhim R., Martincorena I., Pedersen J. S., Getz G.; PCAWG Consortium , Analyses of non-coding somatic drivers in 2,658 cancer whole genomes. Nature 578, 102–111 (2020).
-
- Cohen A. J., Saiakhova A., Corradin O., Luppino J. M., Lovrenert K., Bartels C. F., Morrow J. J., Mack S. C., Dhillon G., Beard L., Myeroff L., Kalady M. F., Willis J., Bradner J. E., Keri R. A., Berger N. A., Pruett-Miller S. M., Markowitz S. D., Scacheri P. C., Hotspots of aberrant enhancer activity punctuate the colorectal cancer epigenome. Nat. Commun. 8, 14400 (2017). - PMC - PubMed
-
- Chapuy B., McKeown M. R., Lin C. Y., Monti S., Roemer M. G. M., Qi J., Rahl P. B., Sun H. H., Yeda K. T., Doench J. G., Reichert E., Kung A. L., Rodig S. J., Young R. A., Shipp M. A., Bradner J. E., Discovery and characterization of super-enhancer-associated dependencies in diffuse large B cell lymphoma. Cancer Cell 24, 777–790 (2013). - PMC - PubMed
-
- Roe J.-S., Hwang C.-I., Somerville T. D. D., Milazzo J. P., Lee E. J., Da Silva B., Maiorino L., Tiriac H., Young C. M., Miyabayashi K., Filippini D., Creighton B., Burkhart R. A., Buscaglia J. M., Kim E. J., Grem J. L., Lazenby A. J., Grunkemeyer J. A., Hollingsworth M. A., Grandgenett P. M., Egeblad M., Park Y., Tuveson D. A., Vakoc C. R., Enhancer reprogramming promotes pancreatic cancer metastasis. Cell 170, 875–888.e20 (2017). - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

