Repression of mRNA translation initiation by GIGYF1 via disrupting the eIF3-eIF4G1 interaction
- PMID: 39018414
- PMCID: PMC466957
- DOI: 10.1126/sciadv.adl5638
Repression of mRNA translation initiation by GIGYF1 via disrupting the eIF3-eIF4G1 interaction
Abstract
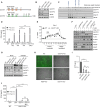
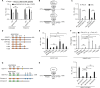
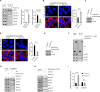
Viruses can selectively repress the translation of mRNAs involved in the antiviral response. RNA viruses exploit the Grb10-interacting GYF (glycine-tyrosine-phenylalanine) proteins 2 (GIGYF2) and eukaryotic translation initiation factor 4E (eIF4E) homologous protein 4EHP to selectively repress the translation of transcripts such as Ifnb1, which encodes the antiviral cytokine interferon-β (IFN-β). Herein, we reveal that GIGYF1, a paralog of GIGYF2, robustly represses cellular mRNA translation through a distinct 4EHP-independent mechanism. Upon recruitment to a target mRNA, GIGYF1 binds to subunits of eukaryotic translation initiation factor 3 (eIF3) at the eIF3-eIF4G1 interaction interface. This interaction disrupts the eIF3 binding to eIF4G1, resulting in transcript-specific translational repression. Depletion of GIGYF1 induces a robust immune response by derepressing IFN-β production. Our study highlights a unique mechanism of translational regulation by GIGYF1 that involves sequestering eIF3 and abrogating its binding to eIF4G1. This mechanism has profound implications for the host response to viral infections.
Figures



References
-
- Grzmil M., Rzymski T., Milani M., Harris A. L., Capper R. G., Saunders N. J., Salhan A., Ragoussis J., Norbury C. J., An oncogenic role of eIF3e/INT6 in human breast cancer. Oncogene 29, 4080–4089 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

