Circ-0006332 stimulates cardiomyocyte pyroptosis via the miR-143/TLR2 axis to promote doxorubicin-induced cardiac damage
- PMID: 39018487
- PMCID: PMC11259061
- DOI: 10.1080/15592294.2024.2380145
Circ-0006332 stimulates cardiomyocyte pyroptosis via the miR-143/TLR2 axis to promote doxorubicin-induced cardiac damage
Abstract
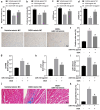
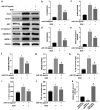
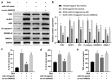
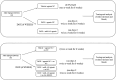
Doxorubicin (DOX)-mediated cardiotoxicity can impair the clinical efficacy of chemotherapy, leading to heart failure (HF). Given the importance of circRNAs and miRNAs in HF, this paper intended to delineate the mechanism of the circular RNA 0006332 (circ -0,006,332)/microRNA (miR)-143/Toll-like receptor 2 (TLR2) axis in doxorubicin (DOX)-induced HF. The binding of miR-143 to circ -0,006,332 and TLR2 was assessed with the dual-luciferase assay, and the binding between miR-143 and circ -0,006,332 was determined with FISH, RIP, and RNA pull-down assays. miR-143 and/or circ -0,006,332 were overexpressed in rats and cardiomyocytes, followed by DOX treatment. In cardiomyocytes, miR-143 and TLR2 expression, cell viability, LDH release, ATP contents, and levels of IL-1β, IL-18, TNF-α, and pyroptosis-related molecules were examined. In rats, cardiac function, serum levels of cardiac enzymes, apoptosis, myocardial fibrosis, and levels of IL-1β, IL-18, TNF-α, TLR2, and pyroptosis-related molecules were detected. miR-143 diminished TLR2 expression by binding to TLR2, and circ -0,006,332 bound to miR-143 to downregulate miR-143 expression. miR-143 expression was reduced and TLR2 expression was augmented in DOX-induced cardiomyocytes. miR-143 inhibited DOX-induced cytotoxicity by suppressing pyroptosis in H9C2 cardiomyocytes. In DOX-induced rats, miR-143 reduced cardiac dysfunction, myocardial apoptosis, myocardial fibrosis, TLR2 levels, and pyroptosis. Furthermore, overexpression of circ -0,006,332 blocked these effects of miR-143 on DOX-induced cardiomyocytes and rats. Circ -0,006,332 stimulates cardiomyocyte pyroptosis by downregulating miR-143 and upregulating TLR2, thus promoting DOX-induced cardiac injury.
Keywords: TLR2; circular RNA-0006332; doxorubicin; heart failure; microRNA-143; pyroptosis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
