Distinct functional and molecular profiles between physiological and pathological atrial enlargement offer potential new therapeutic opportunities for atrial fibrillation
- PMID: 39018488
- PMCID: PMC11292366
- DOI: 10.1042/CS20240178
Distinct functional and molecular profiles between physiological and pathological atrial enlargement offer potential new therapeutic opportunities for atrial fibrillation
Abstract
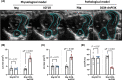
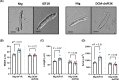
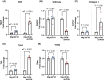
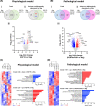
Atrial fibrillation (AF) remains challenging to prevent and treat. A key feature of AF is atrial enlargement. However, not all atrial enlargement progresses to AF. Atrial enlargement in response to physiological stimuli such as exercise is typically benign and reversible. Understanding the differences in atrial function and molecular profile underpinning pathological and physiological atrial remodelling will be critical for identifying new strategies for AF. The discovery of molecular mechanisms responsible for pathological and physiological ventricular hypertrophy has uncovered new drug targets for heart failure. Studies in the atria have been limited in comparison. Here, we characterised mouse atria from (1) a pathological model (cardiomyocyte-specific transgenic (Tg) that develops dilated cardiomyopathy [DCM] and AF due to reduced protective signalling [PI3K]; DCM-dnPI3K), and (2) a physiological model (cardiomyocyte-specific Tg with an enlarged heart due to increased insulin-like growth factor 1 receptor; IGF1R). Both models presented with an increase in atrial mass, but displayed distinct functional, cellular, histological and molecular phenotypes. Atrial enlargement in the DCM-dnPI3K Tg, but not IGF1R Tg, was associated with atrial dysfunction, fibrosis and a heart failure gene expression pattern. Atrial proteomics identified protein networks related to cardiac contractility, sarcomere assembly, metabolism, mitochondria, and extracellular matrix which were differentially regulated in the models; many co-identified in atrial proteomics data sets from human AF. In summary, physiological and pathological atrial enlargement are associated with distinct features, and the proteomic dataset provides a resource to study potential new regulators of atrial biology and function, drug targets and biomarkers for AF.
Keywords: biochemical techniques and resources; cardiac arrhythmia; drug discovery and design; myocardium; proteomics.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures



Similar articles
-
Pathways to precision medicine: deciphering the secrets of physiological and pathological atrial enlargement.Clin Sci (Lond). 2024 Sep 18;138(18):1173-1177. doi: 10.1042/CS20241421. Clin Sci (Lond). 2024. PMID: 39289952
-
Reduced phosphoinositide 3-kinase (p110alpha) activation increases the susceptibility to atrial fibrillation.Am J Pathol. 2009 Sep;175(3):998-1009. doi: 10.2353/ajpath.2009.090126. Epub 2009 Aug 13. Am J Pathol. 2009. PMID: 19679877 Free PMC article.
-
Atrial fibrillation and heart failure-associated remodeling of two-pore-domain potassium (K2P) channels in murine disease models: focus on TASK-1.Basic Res Cardiol. 2018 Jun 7;113(4):27. doi: 10.1007/s00395-018-0687-9. Basic Res Cardiol. 2018. PMID: 29881975
-
The molecular and functional identities of atrial cardiomyocytes in health and disease.Biochim Biophys Acta. 2016 Jul;1863(7 Pt B):1882-93. doi: 10.1016/j.bbamcr.2015.11.025. Epub 2015 Nov 24. Biochim Biophys Acta. 2016. PMID: 26620800 Review.
-
Interleukin-6: Molecular Mechanisms and Therapeutic Perspectives in Atrial Fibrillation.Curr Med Sci. 2025 Apr;45(2):157-168. doi: 10.1007/s11596-025-00021-7. Epub 2025 Mar 4. Curr Med Sci. 2025. PMID: 40035997 Review.
Cited by
-
Genetic and Molecular Underpinnings of Atrial Fibrillation.NPJ Cardiovasc Health. 2024;1:35. doi: 10.1038/s44325-024-00035-5. Epub 2024 Dec 4. NPJ Cardiovasc Health. 2024. PMID: 39867228 Free PMC article.
References
-
- Fuster V., Ryden L.E., Cannom D.S., Crijns H.J., Curtis A.B., Ellenbogen K.A.et al. . (2011) 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J. Am. Coll. Cardiol. 57, e101–e198 10.1016/j.jacc.2010.09.013 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

