Protective Non-neutralizing anti-N-terminal Domain mAb Maintains Fc-mediated Function against SARS-COV-2 Variants up to BA.2.86-JN.1 with Superfluous In Vivo Protection against JN.1 Due to Attenuated Virulence
- PMID: 39018495
- PMCID: PMC11335326
- DOI: 10.4049/jimmunol.2300675
Protective Non-neutralizing anti-N-terminal Domain mAb Maintains Fc-mediated Function against SARS-COV-2 Variants up to BA.2.86-JN.1 with Superfluous In Vivo Protection against JN.1 Due to Attenuated Virulence
Abstract
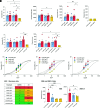
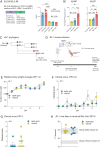
Substantial evidence supports that Fc-mediated effector functions of anti-spike Abs contribute to anti-SARS-Cov-2 protection. We have previously shown that two non-neutralizing but opsonic mAbs targeting the receptor-binding domain and N-terminal domain (NTD), Ab81 and Ab94, respectively, are protective against lethal Wuhan SARS-CoV-2 infection in K18-hACE2 mice. In this article, we investigated whether these protective non-neutralizing Abs maintain Fc-mediated function and Ag binding against mutated SARS-CoV-2 variants. Ab81 and Ab94 retained their nanomolar affinity and Fc-mediated function toward Omicron and its subvariants, such as BA.2, BA.4, BA.5, XBB, XBB1.5, and BQ1.1. However, when encountering the more heavily mutated BA.2.86, Ab81 lost its function, whereas the 10 new mutations in the NTD did not affect Ab94. In vivo experiments with Ab94 in K18-hACE2 mice inoculated with a stringent dose of 100,000 PFU of the JN.1 variant revealed unexpected results. Surprisingly, this variant exhibited low disease manifestation in this animal model with no weight loss or death in the control group. Still, assessment of mice using a clinical scoring system showed better protection for Ab94-treated mice, indicating that Fc-mediated functions are still beneficial. Our work shows that a protective anti-receptor-binding domain non-neutralizing mAb lost reactivity when BA.2.86 emerged, whereas the anti-NTD mAb was still functional. Finally, this work adds new insight into the evolution of the SARS-CoV-2 virus by reporting that JN.1 is substantially less virulent in vivo than previous strains.
Copyright © 2024 by The American Association of Immunologists, Inc.
Conflict of interest statement
A.I. and P.N. have filed a patent pending for the Abs described in this article. The other authors have no financial conflicts of interest.
Figures



References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

