Tailored Dose-Dense Versus Standard Adjuvant Chemotherapy for High-Risk Early Breast Cancer: End-of-Study Results of the Randomized PANTHER Trial
- PMID: 39018515
- PMCID: PMC11379357
- DOI: 10.1200/JCO.24.00178
Tailored Dose-Dense Versus Standard Adjuvant Chemotherapy for High-Risk Early Breast Cancer: End-of-Study Results of the Randomized PANTHER Trial
Abstract
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.Although dose-dense adjuvant chemotherapy administered once every 2 weeks leads to superior outcomes compared with standard regimens once every 3 weeks, the observed improvement is largely limited to studies using the suboptimal paclitaxel schedule once every 3 weeks as control. PANTHER is an international phase III trial which compared sequential epirubicin/cyclophosphamide and docetaxel administered either once every 2 or once every 3 weeks, with tailored dosing at the dose-dense schedule according to hematologic toxicity. In this end-of-study analysis, the median follow-up was 10.3 years. Compared with standard adjuvant chemotherapy, dose-dense treatment improved breast cancer recurrence-free survival (hazard ratio [HR], 0.80 [95% CI, 0.65 to 0.98]; P = .030), event-free survival (HR, 0.78 [95% CI, 0.65 to 0.94]; P = .009), and distant disease-free survival (HR, 0.79 [95% CI, 0.64 to 0.98]; P = .030) while the improvement in overall survival was not statistically significant (HR, 0.82 [95% CI, 0.65 to 1.04]; P = .109). To our knowledge, this is the first trial that confirms the benefit of a dose-dense regimen over a control regimen containing docetaxel once every 3 weeks.
Trial registration: ClinicalTrials.gov NCT00798070.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
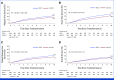
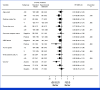
Comment in
-
Dose-dense adjuvant chemotherapy for high-risk early breast cancer: its role in the era of personalised oncology.Gland Surg. 2025 Apr 30;14(4):791-796. doi: 10.21037/gs-2025-72. Epub 2025 Apr 25. Gland Surg. 2025. PMID: 40405947 Free PMC article. No abstract available.
References
-
- Early Breast Cancer Trialists' Collaborative Group EBCTCG : Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: A patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet 393:1440-1452, 2019 - PMC - PubMed
-
- Del Mastro L, De Placido S, Bruzzi P, et al. : Fluorouracil and dose-dense chemotherapy in adjuvant treatment of patients with early-stage breast cancer: An open-label, 2×2 factorial, randomised phase 3 trial. Lancet 385:1863-1872, 2015 - PubMed
-
- Citron ML, Berry DA, Cirrincione C, et al. : Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of intergroup trial C9741/cancer and leukemia group B trial 9741. J Clin Oncol 21:1431-1439, 2003 - PubMed
-
- Foukakis T, von Minckwitz G, Bengtsson NO, et al. : Effect of tailored dose-dense chemotherapy vs standard 3-weekly adjuvant chemotherapy on recurrence-free survival among women with high-risk early breast cancer: A randomized clinical trial. JAMA 316:1888-1896, 2016 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

