Detection of Common Respiratory Infections, Including COVID-19, Using Consumer Wearable Devices in Health Care Workers: Prospective Model Validation Study
- PMID: 39018555
- PMCID: PMC11292157
- DOI: 10.2196/53716
Detection of Common Respiratory Infections, Including COVID-19, Using Consumer Wearable Devices in Health Care Workers: Prospective Model Validation Study
Abstract
Background: The early detection of respiratory infections could improve responses against outbreaks. Wearable devices can provide insights into health and well-being using longitudinal physiological signals.
Objective: The purpose of this study was to prospectively evaluate the performance of a consumer wearable physiology-based respiratory infection detection algorithm in health care workers.
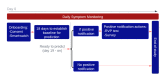
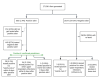
Methods: In this study, we evaluated the performance of a previously developed system to predict the presence of COVID-19 or other upper respiratory infections. The system generates real-time alerts using physiological signals recorded from a smartwatch. Resting heart rate, respiratory rate, and heart rate variability measured during the sleeping period were used for prediction. After baseline recordings, when participants received a notification from the system, they were required to undergo testing at a Northwell Health System site. Participants were asked to self-report any positive tests during the study. The accuracy of model prediction was evaluated using respiratory infection results (laboratory results or self-reports), and postnotification surveys were used to evaluate potential confounding factors.
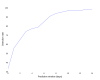
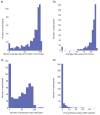
Results: A total of 577 participants from Northwell Health in New York were enrolled in the study between January 6, 2022, and July 20, 2022. Of these, 470 successfully completed the study, 89 did not provide sufficient physiological data to receive any prediction from the model, and 18 dropped out. Out of the 470 participants who completed the study and wore the smartwatch as required for the 16-week study duration, the algorithm generated 665 positive alerts, of which 153 (23.0%) were not acted upon to undergo testing for respiratory viruses. Across the 512 instances of positive alerts that involved a respiratory viral panel test, 63 had confirmed respiratory infection results (ie, COVID-19 or other respiratory infections detected using a polymerase chain reaction or home test) and the remaining 449 had negative upper respiratory infection test results. Across all cases, the estimated false-positive rate based on predictions per day was 2%, and the positive-predictive value ranged from 4% to 10% in this specific population, with an observed incidence rate of 198 cases per week per 100,000. Detailed examination of questionnaires filled out after receiving a positive alert revealed that physical or emotional stress events, such as intense exercise, poor sleep, stress, and excessive alcohol consumption, could cause a false-positive result.
Conclusions: The real-time alerting system provides advance warning on respiratory viral infections as well as other physical or emotional stress events that could lead to physiological signal changes. This study showed the potential of wearables with embedded alerting systems to provide information on wellness measures.
Keywords: COVID detection; COVID-19; algorithm; emotional stress; health; health care worker; infection; physical stress; physiology; prediction; respiratory infection; respiratory virus; respiratory virus detection; wearable; wearable device; well-being.
©Zeinab Esmaeilpour, Aravind Natarajan, Hao-Wei Su, Anthony Faranesh, Ciaran Friel, Theodoros P Zanos, Stefani D’Angelo, Conor Heneghan. Originally published in JMIR Formative Research (https://formative.jmir.org), 17.07.2024.
Conflict of interest statement
Conflicts of Interest: ZE, AN, HWS, AF, and CH are employees of Google and own shares in Alphabet Inc. CF has a family member who owns stock in Google, which could potentially benefit from the outcomes of this research. Google purchased Fitbit during the course of the study. TZ and SD do not have any competing interests to disclose.
Figures
Similar articles
-
Real-time alerting system for COVID-19 and other stress events using wearable data.Nat Med. 2022 Jan;28(1):175-184. doi: 10.1038/s41591-021-01593-2. Epub 2021 Nov 29. Nat Med. 2022. PMID: 34845389 Free PMC article.
-
Feasibility of wearable sensor signals and self-reported symptoms to prompt at-home testing for acute respiratory viruses in the USA (DETECT-AHEAD): a decentralised, randomised controlled trial.Lancet Digit Health. 2024 Aug;6(8):e546-e554. doi: 10.1016/S2589-7500(24)00096-7. Lancet Digit Health. 2024. PMID: 39059887 Free PMC article. Clinical Trial.
-
Virtualized clinical studies to assess the natural history and impact of gut microbiome modulation in non-hospitalized patients with mild to moderate COVID-19 a randomized, open-label, prospective study with a parallel group study evaluating the physiologic effects of KB109 on gut microbiota structure and function: a structured summary of a study protocol for a randomized controlled study.Trials. 2021 Apr 2;22(1):245. doi: 10.1186/s13063-021-05157-0. Trials. 2021. PMID: 33810796 Free PMC article.
-
[Standard technical specifications for methacholine chloride (Methacholine) bronchial challenge test (2023)].Zhonghua Jie He He Hu Xi Za Zhi. 2024 Feb 12;47(2):101-119. doi: 10.3760/cma.j.cn112147-20231019-00247. Zhonghua Jie He He Hu Xi Za Zhi. 2024. PMID: 38309959 Chinese.
-
Universal screening for SARS-CoV-2 infection: a rapid review.Cochrane Database Syst Rev. 2020 Sep 15;9(9):CD013718. doi: 10.1002/14651858.CD013718. Cochrane Database Syst Rev. 2020. PMID: 33502003 Free PMC article.
Cited by
-
The Value of Smartwatches in the Health Care Sector for Monitoring, Nudging, and Predicting: Viewpoint on 25 Years of Research.J Med Internet Res. 2024 Oct 25;26:e58936. doi: 10.2196/58936. J Med Internet Res. 2024. PMID: 39356287 Free PMC article. Review.
References
-
- Holko M, Litwin TR, Munoz F, Theisz KI, Salgin L, Jenks NP, Holmes BW, Watson-McGee P, Winford E, Sharma Y. Wearable fitness tracker use in federally qualified health center patients: strategies to improve the health of all of us using digital health devices. NPJ Digit Med. 2022 Apr 25;5(1):53. doi: 10.1038/s41746-022-00593-x. doi: 10.1038/s41746-022-00593-x.10.1038/s41746-022-00593-x - DOI - DOI - PMC - PubMed
-
- Sun S, Folarin AA, Ranjan Y, Rashid Z, Conde P, Stewart C, Cummins N, Matcham F, Dalla Costa G, Simblett S, Leocani L, Lamers F, Sørensen P, Buron M, Zabalza A, Guerrero Pérez A, Penninx BW, Siddi S, Haro JM, Myin-Germeys I, Rintala A, Wykes T, Narayan VA, Comi G, Hotopf M, Dobson RJ, RADAR-CNS Consortium Using smartphones and wearable devices to monitor behavioral changes during COVID-19. J Med Internet Res. 2020 Sep 25;22(9):e19992. doi: 10.2196/19992. https://www.jmir.org/2020/9/e19992/ v22i9e19992 - DOI - PMC - PubMed
-
- Hadid A, McDonald EG, Cheng MP, Papenburg J, Libman M, Dixon PC, Jensen D. The WE SENSE study protocol: A controlled, longitudinal clinical trial on the use of wearable sensors for early detection and tracking of viral respiratory tract infections. Contemp Clin Trials. 2023 May;128:107103. doi: 10.1016/j.cct.2023.107103. https://europepmc.org/abstract/MED/37147083 S1551-7144(23)00026-5 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

