Policy Guidance for Direct-to-Consumer Genetic Testing Services: Framework Development Study
- PMID: 39018558
- PMCID: PMC11292153
- DOI: 10.2196/47389
Policy Guidance for Direct-to-Consumer Genetic Testing Services: Framework Development Study
Abstract
Background: The online offer of commercial genetic tests, also called direct-to-consumer genetic tests (DTC-GTs), enables citizens to gain insight into their health and disease risk based on their genetic profiles. DTC-GT offers often consist of a combination of services or aspects, including advertisements, information, DNA analysis, and medical or lifestyle advice. The risks and benefits of DTC-GT services have been debated and studied extensively, but instruments that assess DTC-GT services and aid policy are lacking. This leads to uncertainty among policy makers, law enforcers, and regulators on how to ensure and balance both public safety and autonomy and about the responsibilities these 3 parties have toward the public.
Objective: This study aimed to develop a framework that outlines aspects of DTC-GTs that lead to policy issues and to help provide policy guidance regarding DTC-GT services.
Methods: We performed 3 steps: (1) an integrative literature review to identify risks and benefits of DTC-GT services for consumers and society in Embase and Medline (January 2014-June 2022), (2) structuring benefits and risks in different steps of the consumer journey, and (3) development of a checklist for policy guidance.
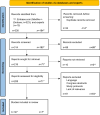
Results: Potential risks and benefits of DTC-GT services were mapped from 134 papers and structured into 6 phases. In summary, these phases were called the consumer journey: (1) exposure, (2) pretest information, (3) DNA analysis, (4) data management, (5) posttest information, and (6) individual and societal impact. The checklist for evaluation of DTC-GT services consisted of 8 themes, covering 38 items that may raise policy issues in DTC-GT services. The themes included the following aspects: general service content, validity and quality assurance, potential data and privacy risks, scientific evidence and robustness, and quality of the provided information.
Conclusions: Both the consumer journey and the checklist break the DTC-GT offer down into key aspects that may impact and compromise individual and public health, safety, and autonomy. This framework helps policy makers, regulators, and law enforcers develop methods to interpret, assess, and act in the DTC-GT service market.
Keywords: direct-to-consumer testing; genetic privacy; genetic testing; health policy; informed consent; mHealth; mobile health; online market; policy decision; privacy; public health genomics.
©Suzanne Maria Onstwedder, Marleen Elizabeth Jansen, Martina Cornelia Cornel, Tessel Rigter. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 17.07.2024.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures


References
-
- Wetterstrand K. DNA sequencing costs: data from the NHGRI Genome Sequencing Program (GSP) National Human Genome Research Institute. 2016. [2024-06-21]. https://www.genome.gov/sequencingcostsdata/
-
- Thiebes S, Toussaint PA, Ju J, Ahn J, Lyytinen K, Sunyaev A. Valuable genomes: taxonomy and archetypes of business models in direct-to-consumer genetic testing. J Med Internet Res. 2020 Jan 21;22(1):e14890. doi: 10.2196/14890. https://www.jmir.org/2020/1/e14890/ v22i1e14890 - DOI - PMC - PubMed
-
- Badalato L, Kalokairinou L, Borry P. Third party interpretation of raw genetic data: an ethical exploration. Eur J Hum Genet. 2017 Nov 23;25(11):1189–1194. doi: 10.1038/ejhg.2017.126. https://europepmc.org/abstract/MED/28832567 ejhg2017126 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

