UBXN3B is crucial for B lymphopoiesis
- PMID: 39018756
- PMCID: PMC11287013
- DOI: 10.1016/j.ebiom.2024.105248
UBXN3B is crucial for B lymphopoiesis
Abstract
Background: The ubiquitin regulatory X (UBX) domain-containing proteins (UBXNs) are putative adaptors for ubiquitin ligases and valosin-containing protein; however, their in vivo physiological functions remain poorly characterised. We recently showed that UBXN3B is essential for activating innate immunity to DNA viruses and controlling DNA/RNA virus infection. Herein, we investigate its role in adaptive immunity.
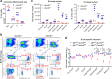
Methods: We evaluated the antibody responses to multiple viruses and pathogenesis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and influenza in tamoxifen-inducible global and constitutive B cell-specific Ubxn3b knockout mice; quantified various immune populations, B lineage progenitors/precursors, B cell receptor (BCR) signalling and apoptosis by flow cytometry, immunoblotting and immunofluorescence microscopy. We also performed bone marrow transfer, single-cell and bulk RNA sequencing.
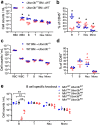
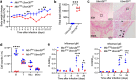
Findings: Both global and B cell-specific Ubxn3b knockout mice present a marked reduction in small precursor B-II (>60%), immature (>70%) and mature B (>95%) cell numbers. Transfer of wildtype bone marrow to irradiated global Ubxn3b knockouts restores normal B lymphopoiesis, while reverse transplantation does not. The mature B population shrinks rapidly with apoptosis and higher pro and activated caspase-3 protein levels were observed following induction of Ubxn3b knockout. Mechanistically, Ubxn3b deficiency leads to impaired pre-BCR signalling and cell cycle arrest. Ubxn3b knockout mice are highly vulnerable to respiratory viruses, with increased viral loads and prolonged immunopathology in the lung, and reduced production of virus-specific IgM/IgG.
Interpretation: UBXN3B is essential for B lymphopoiesis by maintaining constitutive pre-BCR signalling and cell survival in a cell-intrinsic manner.
Funding: United States National Institutes of Health grants, R01AI132526 and R21AI155820.
Keywords: B cell; COVID-19; Haematopoiesis; Lymphopoiesis; UBXN.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests No financial or non-financial interest to disclose.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

