PARP11 inhibition inactivates tumor-infiltrating regulatory T cells and improves the efficacy of immunotherapies
- PMID: 39019005
- PMCID: PMC11293321
- DOI: 10.1016/j.xcrm.2024.101649
PARP11 inhibition inactivates tumor-infiltrating regulatory T cells and improves the efficacy of immunotherapies
Abstract
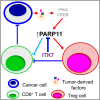
Tumor-infiltrating regulatory T cells (TI-Tregs) elicit immunosuppressive effects in the tumor microenvironment (TME) leading to accelerated tumor growth and resistance to immunotherapies against solid tumors. Here, we demonstrate that poly-(ADP-ribose)-polymerase-11 (PARP11) is an essential regulator of immunosuppressive activities of TI-Tregs. Expression of PARP11 correlates with TI-Treg cell numbers and poor responses to immune checkpoint blockade (ICB) in human patients with cancer. Tumor-derived factors including adenosine and prostaglandin E2 induce PARP11 in TI-Tregs. Knockout of PARP11 in the cells of the TME or treatment of tumor-bearing mice with selective PARP11 inhibitor ITK7 inactivates TI-Tregs and reinvigorates anti-tumor immune responses. Accordingly, ITK7 decelerates tumor growth and significantly increases the efficacy of anti-tumor immunotherapies including ICB and adoptive transfer of chimeric antigen receptor (CAR) T cells. These results characterize PARP11 as a key driver of TI-Treg activities and a major regulator of immunosuppressive TME and argue for targeting PARP11 to augment anti-cancer immunotherapies.
Keywords: ITK7; PARP11; PARP11 inhibitor; Treg cells; immunotherapy; tumor microenvironment.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.S.C. is an inventor on an OHSU patent related to the general use of the ITK7 compound. M.S.C., R.B., and S.Y.F. are co-inventors on a pending patent from OHSU/University of Pennsylvania related to ITK7 use.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

