Neddylation inhibition prevents acetaminophen-induced liver damage by enhancing the anabolic cardiolipin pathway
- PMID: 39019009
- PMCID: PMC11293357
- DOI: 10.1016/j.xcrm.2024.101653
Neddylation inhibition prevents acetaminophen-induced liver damage by enhancing the anabolic cardiolipin pathway
Abstract
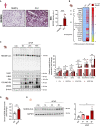
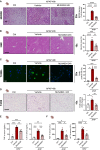
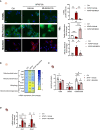
Drug-induced liver injury (DILI) is a significant cause of acute liver failure (ALF) and liver transplantation in the Western world. Acetaminophen (APAP) overdose is a main contributor of DILI, leading to hepatocyte cell death through necrosis. Here, we identified that neddylation, an essential post-translational modification involved in the mitochondria function, was upregulated in liver biopsies from patients with APAP-induced liver injury (AILI) and in mice treated with an APAP overdose. MLN4924, an inhibitor of the neuronal precursor cell-expressed developmentally downregulated protein 8 (NEDD8)-activating enzyme (NAE-1), ameliorated necrosis and boosted liver regeneration in AILI. To understand how neddylation interferes in AILI, whole-body biotinylated NEDD8 (bioNEDD8) and ubiquitin (bioUB) transgenic mice were investigated under APAP overdose with and without MLN4924. The cytidine diphosphate diacylglycerol (CDP-DAG) synthase TAM41, responsible for producing cardiolipin essential for mitochondrial activity, was found modulated under AILI and restored its levels by inhibiting neddylation. Understanding this ubiquitin-like crosstalk in AILI is essential for developing promising targeted inhibitors for DILI treatment.
Keywords: APAP; DILI; NEDD8; mitochondria; necrosis.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

