Aberrant bowel movement frequencies coincide with increased microbe-derived blood metabolites associated with reduced organ function
- PMID: 39019013
- PMCID: PMC11293344
- DOI: 10.1016/j.xcrm.2024.101646
Aberrant bowel movement frequencies coincide with increased microbe-derived blood metabolites associated with reduced organ function
Abstract
Bowel movement frequency (BMF) directly impacts the gut microbiota and is linked to diseases like chronic kidney disease or dementia. In particular, prior work has shown that constipation is associated with an ecosystem-wide switch from fiber fermentation and short-chain fatty acid production to more detrimental protein fermentation and toxin production. Here, we analyze multi-omic data from generally healthy adults to see how BMF affects their molecular phenotypes, in a pre-disease context. Results show differential abundances of gut microbial genera, blood metabolites, and variation in lifestyle factors across BMF categories. These differences relate to inflammation, heart health, liver function, and kidney function. Causal mediation analysis indicates that the association between lower BMF and reduced kidney function is partially mediated by the microbially derived toxin 3-indoxyl sulfate (3-IS). This result, in a generally healthy context, suggests that the accumulation of microbiota-derived toxins associated with abnormal BMF precede organ damage and may be drivers of chronic, aging-related diseases.
Keywords: bowel movement frequency; chronic disease; gut microbiome; health; lifestyle; protein fermentation; short chain fatty acids.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests L.H. is a former shareholder of Arivale. A.T.M. was a former employee of Arivale. Arivale is no longer a commercially operating company as of April 2019.
Figures

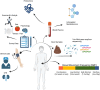
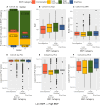
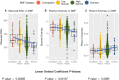
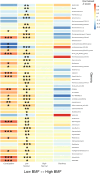


Update of
-
Generally-healthy individuals with aberrant bowel movement frequencies show enrichment for microbially-derived blood metabolites associated with reduced kidney function.bioRxiv [Preprint]. 2024 Mar 9:2023.03.04.531100. doi: 10.1101/2023.03.04.531100. bioRxiv. 2024. Update in: Cell Rep Med. 2024 Jul 16;5(7):101646. doi: 10.1016/j.xcrm.2024.101646. PMID: 36945445 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

