Dietary medium-chain fatty acids reduce hepatic fat accumulation via activation of a CREBH-FGF21 axis
- PMID: 39019116
- PMCID: PMC11327439
- DOI: 10.1016/j.molmet.2024.101991
Dietary medium-chain fatty acids reduce hepatic fat accumulation via activation of a CREBH-FGF21 axis
Abstract
Objective: Dietary medium-chain fatty acids (MCFAs), characterized by chain lengths of 8-12 carbon atoms, have been proposed to have beneficial effects on glucose and lipid metabolism, yet the underlying mechanisms remain elusive. We hypothesized that MCFA intake benefits metabolic health by inducing the release of hormone-like factors.
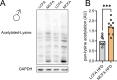
Methods: The effects of chow diet, high-fat diet rich in long-chain fatty acids (LCFA HFD) fed ad libitum or pair-fed to a high-fat diet rich in MCFA (MCFA HFD) on glycemia, hepatic gene expression, circulating fibroblast growth factor 21 (FGF21), and liver fat content in both wildtype and Fgf21 knockout mice were investigated. The impact of a single oral dose of an MCFA-rich oil on circulating FGF21 and hepatic Fgf21 mRNA expression was assessed. In flag-tagged Crebh knockin mice and liver-specific Crebh knockout mice, fed LCFA HFD or MCFA HFD, active hepatic CREBH and hepatic Fgf21 mRNA abundance were determined, respectively.
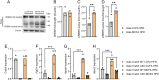
Results: MCFA HFD improves glucose tolerance, enhances glucose clearance into brown adipose tissue, and prevents high-fat diet-induced hepatic steatosis in wildtype mice. These benefits are associated with increased liver expression of CREBH target genes (Apoa4 and Apoc2), including Fgf21. Both acute and chronic intake of dietary MCFAs elevate circulating FGF21. Augmented hepatic Fgf21 mRNA following MCFA HFD intake is accompanied by higher levels of active hepatic CREBH; and MCFA-induced hepatic Fgf21 expression is blocked in mice lacking Crebh. Notably, while feeding male and female Fgf21 wildtype mice MCFA HFD results in reduced liver triacylglycerol (TG) levels, this liver TG-lowering effect is blunted in Fgf21 knockout mice fed MCFA HFD. The reduction in liver TG levels observed with MCFA HFD was independent of weight loss.
Conclusions: Dietary MCFAs reduce liver fat accumulation via activation of a CREBH-FGF21 signaling axis.
Keywords: FGF21; Hepatokines; Insulin resistance; Liver metabolism; Medium-chain fatty acids.
Copyright © 2024 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: C.C. is co-founder of Ousia Pharma ApS, a biotech company developing therapeutics for obesity. C.C. is also on the editorial board of Molecular Metabolism. The remaining authors declare no competing interests.
Figures





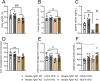
References
-
- Warensjo E., Riserus U., Vessby B. Fatty acid composition of serum lipids predicts the development of the metabolic syndrome in men. Diabetologia. 2005;48(10):1999–2005. - PubMed
-
- Forsythe C.E., Phinney S.D., Fernandez M.L., Quann E.E., Wood R.J., Bibus D.M., et al. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids. 2008;43(1):65–77. - PubMed
-
- King I.B., Lemaitre R.N., Kestin M. Effect of a low-fat diet on fatty acid composition in red cells, plasma phospholipids, and cholesterol esters: investigation of a biomarker of total fat intake. Am J Clin Nutr. 2006;83(2):227–236. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

