Estrogen receptor-α signaling in tanycytes lies at the crossroads of fertility and metabolism
- PMID: 39019342
- PMCID: PMC7616427
- DOI: 10.1016/j.metabol.2024.155976
Estrogen receptor-α signaling in tanycytes lies at the crossroads of fertility and metabolism
Abstract
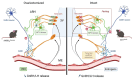
Background: Estrogen secretion by the ovaries regulates the hypothalamic-pituitary-gonadal axis during the reproductive cycle, influencing gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH) secretion, and also plays a role in regulating metabolism. Here, we establish that hypothalamic tanycytes-specialized glia lining the floor and walls of the third ventricle-integrate estrogenic feedback signals from the gonads and couple reproduction with metabolism by relaying this information to orexigenic neuropeptide Y (NPY) neurons.
Methods: Using mouse models, including mice floxed for Esr1 (encoding estrogen receptor alpha, ERα) and those with Cre-dependent expression of designer receptors exclusively activated by designer drugs (DREADDs), along with viral-mediated, pharmacological and indirect calorimetric approaches, we evaluated the role of tanycytes and tanycytic estrogen signaling in pulsatile LH secretion, cFos expression in NPY neurons, estrous cyclicity, body-weight changes and metabolic parameters in adult females.
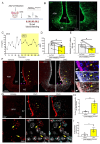
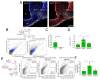
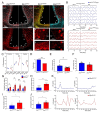
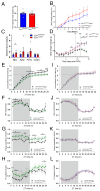
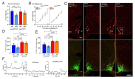
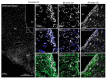
Results: In ovariectomized mice, chemogenetic activation of tanycytes significantly reduced LH pulsatile release, mimicking the effects of direct NPY neuron activation. In intact mice, tanycytes were crucial for the estrogen-mediated control of GnRH/LH release, with tanycytic ERα activation suppressing fasting-induced NPY neuron activation. Selective knockout of Esr1 in tanycytes altered estrous cyclicity and fertility in female mice and affected estrogen's ability to inhibit refeeding in fasting mice. The absence of ERα signaling in tanycytes increased Npy transcripts and body weight in intact mice and prevented the estrogen-mediated decrease in food intake as well as increase in energy expenditure and fatty acid oxidation in ovariectomized mice.
Conclusions: Our findings underscore the pivotal role of tanycytes in the neuroendocrine coupling of reproduction and metabolism, with potential implications for its age-related deregulation after menopause.
Significance statement: Our investigation reveals that tanycytes, specialized glial cells in the brain, are key interpreters of estrogen signals for orexigenic NPY neurons in the hypothalamus. Disrupting tanycytic estrogen receptors not only alters fertility in female mice but also impairs the ability of estrogens to suppress appetite. This work thus sheds light on the critical role played by tanycytes in bridging the hormonal regulation of cyclic reproductive function and appetite/feeding behavior. This understanding may have potential implications for age-related metabolic deregulation after menopause.
Keywords: Estrogens; Female metabolism; Hypothalamus; LH pulsatility; Reproduction; Tanycytes.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no competing interests.
Figures
References
-
- Hunzicker-Dunn M, Mayo K. In: Knobil and Neill’s Physiology of Reproduction. Fourth Edition. Plant TM, Zeleznik J, editors. Elsevier; New York: 2015. Gonadotropinsignaling in the ovary; pp. 895–946.
-
- Sarkar DK, Minami S. Diurnal variation in luteinizing hormone-releasing hormone and beta-endorphin release in pituitary portal plasma during the rat estrous cycle. Biol Reprod. 1995;53:38–45. - PubMed
-
- Caraty A, Orgeur P, Thiery JC. Demonstration of the pulsatile secretion of LH-RH into hypophysial portal blood of ewes using an original technic for multiple samples. C R Seances Acad Sci III. 1982;295:103–6. - PubMed
-
- Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111:1737–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

